Olopatadine hydrochloride nasal spray and preparation method thereof
A technology of olopatadine hydrochloride and nasal spray, applied in the field of medicine, can solve the problems of easy to produce bitter taste, influence on the stability of olopatadine, existence of ciliary toxicity, etc., to improve bioavailability, prolong residence time, improve The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3、 comparative example 1
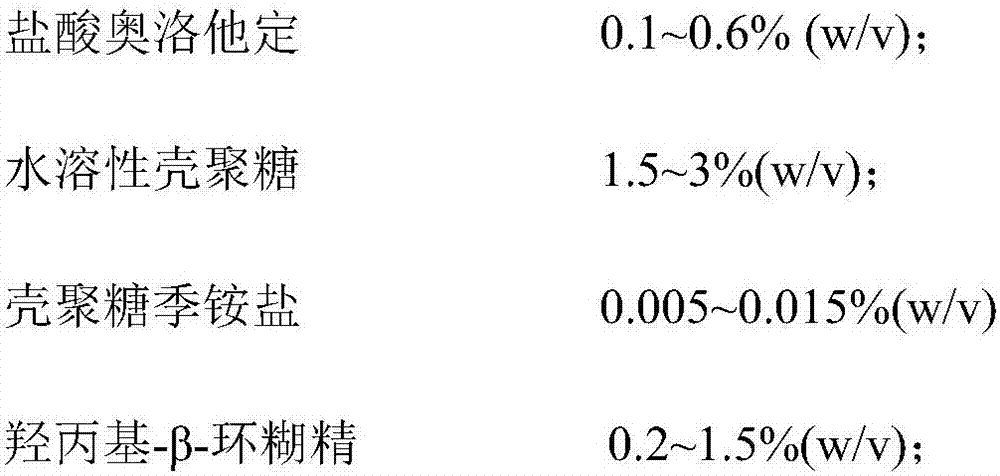
[0029] Preparation of Examples 1-3, Comparative Examples 1-2 Olopatadine Hydrochloride Nasal Spray
[0030] formula:
[0031]
[0032]
[0033] Preparation:
[0034] S1: Take hydroxypropyl-β-cyclodextrin, add purified water to make a saturated solution, add olopatadine hydrochloride, and sonicate for 30 minutes at room temperature to obtain solution I;
[0035] S2: Take water-soluble chitosan, chitosan quaternary ammonium salt and osmotic pressure regulator, add appropriate amount of purified water to dissolve completely, and obtain solution II;
[0036] S3: Stir and mix the solution I described in step S1 and the solution II described in step S2, add purified water to adjust the solution volume to 80% of the final solution volume, add 6mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution to adjust pH , add buffer, add 6mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution to adjust pH, finally add purified water to make the solution volu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com