Cell-nucleus-targeted antitumor nanomedicine carrier and preparation method and application thereof
A nano-drug carrier and cell nucleus technology, applied in the fields of polymer chemistry and biomedical engineering, to prolong the circulation time of body fluids, promote enrichment, and realize the effects of release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
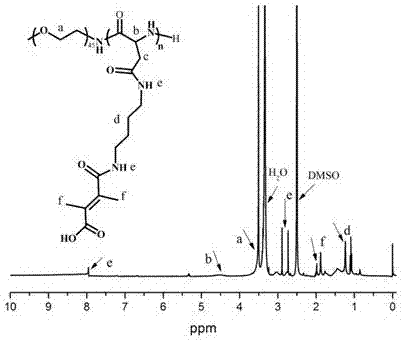
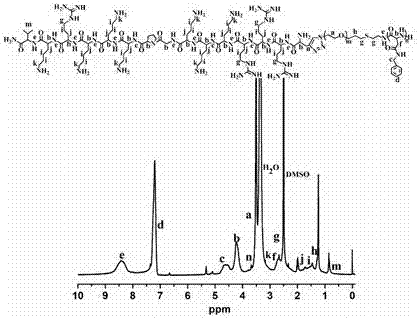
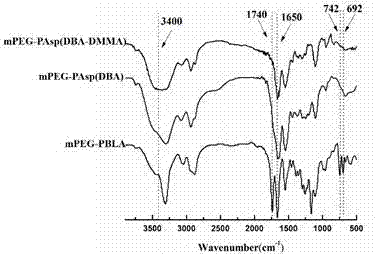
[0041] Example 1 Synthesis of polymer micelles NLS-PEG-PAsp (BzA) and mPEG-PAsp (DBA-DMMA)
[0042] (1) Synthesis of benzyloxycarbonyl aspartic anhydride (BLA-NCA)
[0043]
[0044] Synthesize β-aspartic acid benzyl ester first, and the steps are as follows: add 100 ml of anhydrous diethyl ether into a 500 ml single-mouthed eggplant-type bottle, slowly add 10 ml of concentrated sulfuric acid under stirring, and add 100 ml after the liquid in the bottle returns to room temperature Benzyl alcohol was concentrated by rotary evaporation to remove ether, and 13.3 g of aspartic acid was added to a one-necked bottle, and the reaction was stirred at room temperature for 24 h. After the reaction is complete, add 300 ml of 95% ethanol to the single-necked bottle and shake to mix evenly, and slowly add concentrated NH 3 •H 2 O, gradually produce white precipitate, stop adding concentrated NH when the pH value is adjusted to about 7 3 •H 2 O, the cloudy solution was placed in a refri...
Embodiment 2
[0066] Example 2 Preparation of drug-loaded micelles (NLS-PEG-PAsp(BzA)@DOX)
[0067] Take 20 mg NLS-PEG-PAsp (BzA) dissolved in 3 ml DMSO, another 2.5 mg DOX·HCl dissolved in 1 ml DMSO, and add 60 μl TEA and stir for 2 h. The two were mixed together and slowly dropped into 30 ml of ultrapure water while ultrasonicating, and dialyzed against water for 12 h after completion.
Embodiment 3
[0068] Example 3 Drug Release of NLS-PEG-PAsp(BzA)@DOX
[0069]The conventional dialysis method was used to test the in vitro release experiment to simulate the release of the drug loaded in the micelles. The DOX-loaded polymer micelle solution was divided into three parts on average, and the conditions of the three samples were set at pH 7.4, pH 6.5, and pH 5.0, respectively. The release experiment was carried out in a constant temperature water bath culture shaker at 37 °C. In addition, three parallel groups were set up for each sample. In each group, 2 ml of micellar solution was added to a 14 kDa dialysis bag, and 10 ml of PBS buffer under the same conditions were added to the outside of the dialysis bag for simulated release in a shaker. Then at different set time points (1 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 24 h), the solutions outside the dialysis bags of each group were collected, and the same volume of PBS was added respectively buffer. The absorbance at 480.25 nm o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com