Water-soluble cyclodextrin drug carrier with cell targeting and preparation method thereof
A cell-targeting and cyclodextrin technology, which is used in non-active medical preparations, pharmaceutical formulations, and non-active macromolecular compounds to achieve the effects of strong stereoselectivity, few by-products, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This embodiment provides a water-soluble cyclodextrin drug carrier with cell targeting, which mainly uses β-cyclodextrin (β-CD) as the main body, and introduces at least one phosphorylcholine on it through a click reaction The reverse order structure molecule - phosphorylcholine (CP).
[0043] This example mainly focuses on the single-substituted phosphocholine cyclodextrin with only one phosphorylcholine (CP) introduced into β-cyclodextrin (β-CD). There are multiple fixed-point links on other β-cyclodextrins. The compounds of phosphorylcholine can be obtained based on the preparation process of monosubstituted phosphorylcholine cyclodextrin.
[0044] The specific preparation process of monosubstituted phosphocholine cyclodextrin as a water-soluble cyclodextrin drug carrier with cell targeting comprises the following steps:
[0045] (1) Synthesis of 2-methoxy-2-oxo-1,3,2-dioxophospholane (MDP)
[0046] Glass instruments need to be dried with a flame gun and protected ...
Embodiment 2
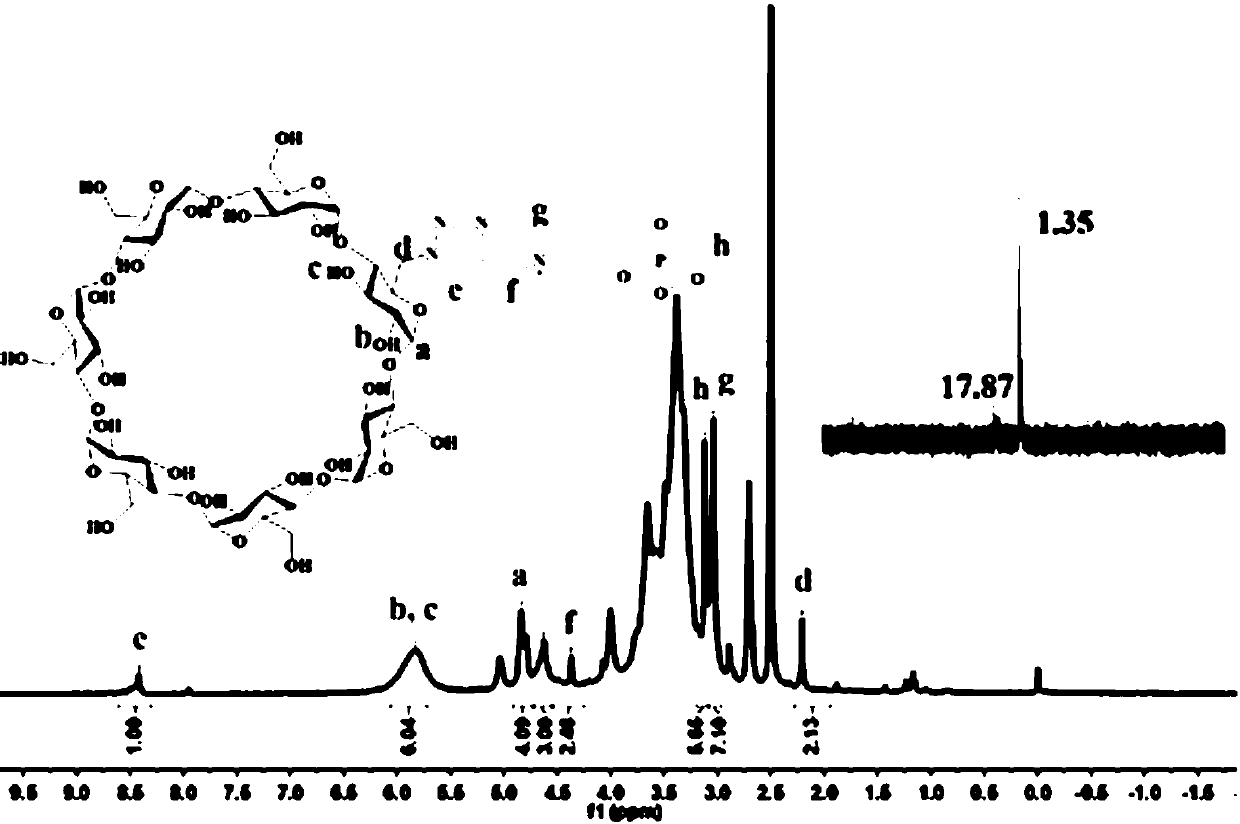
[0055] In this example, the drug carrier (monosubstituted phosphorylcholine cyclodextrin) prepared in the above example was characterized by using nuclear magnetic resonance (400Hz) to characterize the synthetic product, tetramethylsiloxane (TMS) was used as a reference, and the solution was selected DMSO-d 6 or D 2 O, respectively detect the synthesis product 1 HNMR, 31 PNMR.
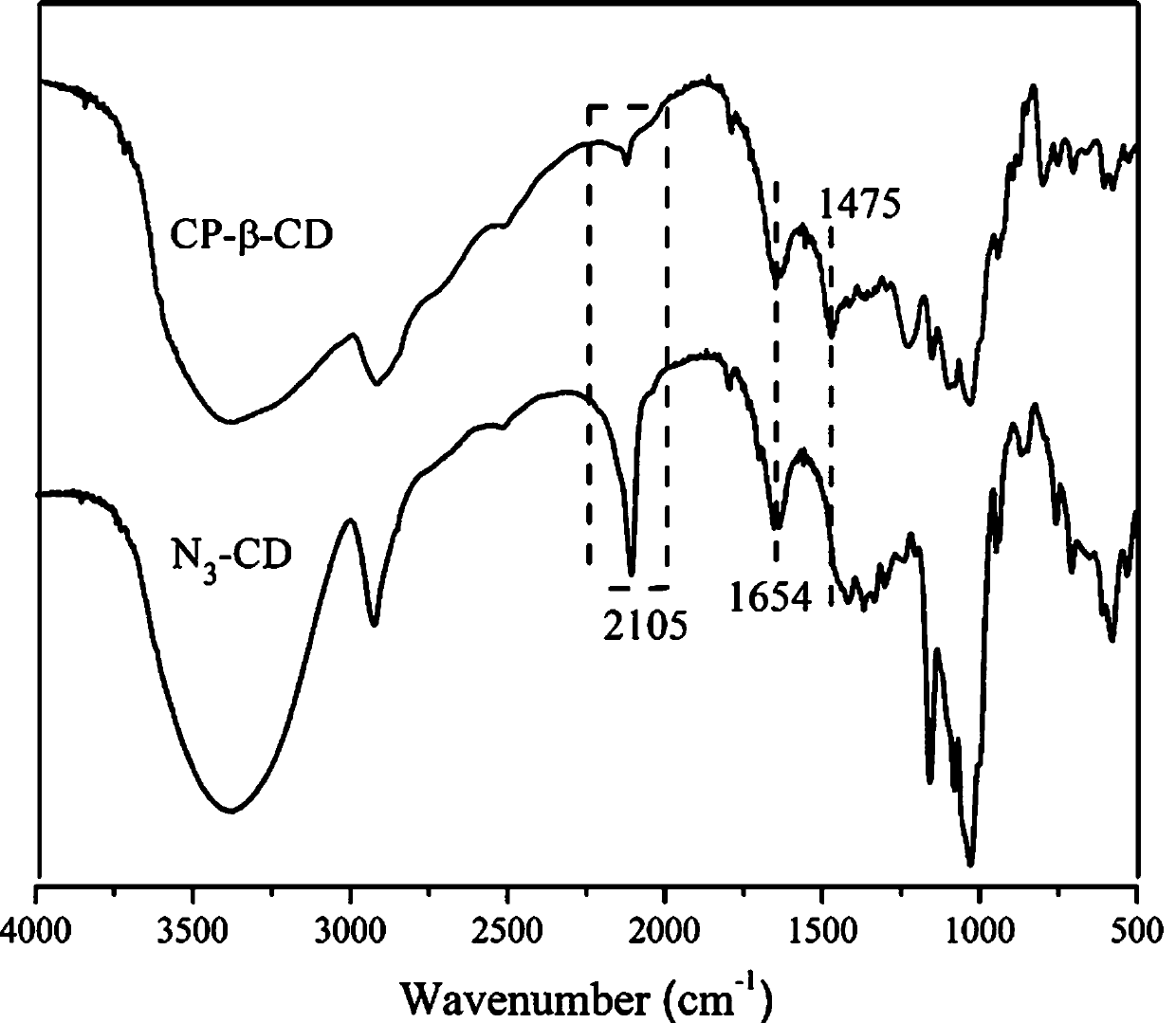
[0056] The prepared phosphocholine-modified cyclodextrin solid is mixed with KBr, ground, pressed into tablets, and scanned in a transmission mode with a wavelength range of 500-4000nm.
[0057] A gas chromatograph is used in conjunction with methanol as a solvent.
[0058] Structural characterization of phosphorylcholine-modified cyclodextrins, as figure 1 As shown in the figure, it can be seen that δ=7.98ppm corresponds to the corresponding hydrogen atoms on the triazole ring, and the characteristic absorption peaks of 14 hydrogen atoms corresponding to cyclodextrin 2 and 3-OH can be seen near 5...
Embodiment 3
[0061] In this example, for the drug carrier (monosubstituted phosphorylcholine cyclodextrin) prepared in the above examples, apatinib was used as a model drug to conduct research on the inclusion capacity of the phosphorylcholine modified cyclodextrin carrier (phase solubility study ).
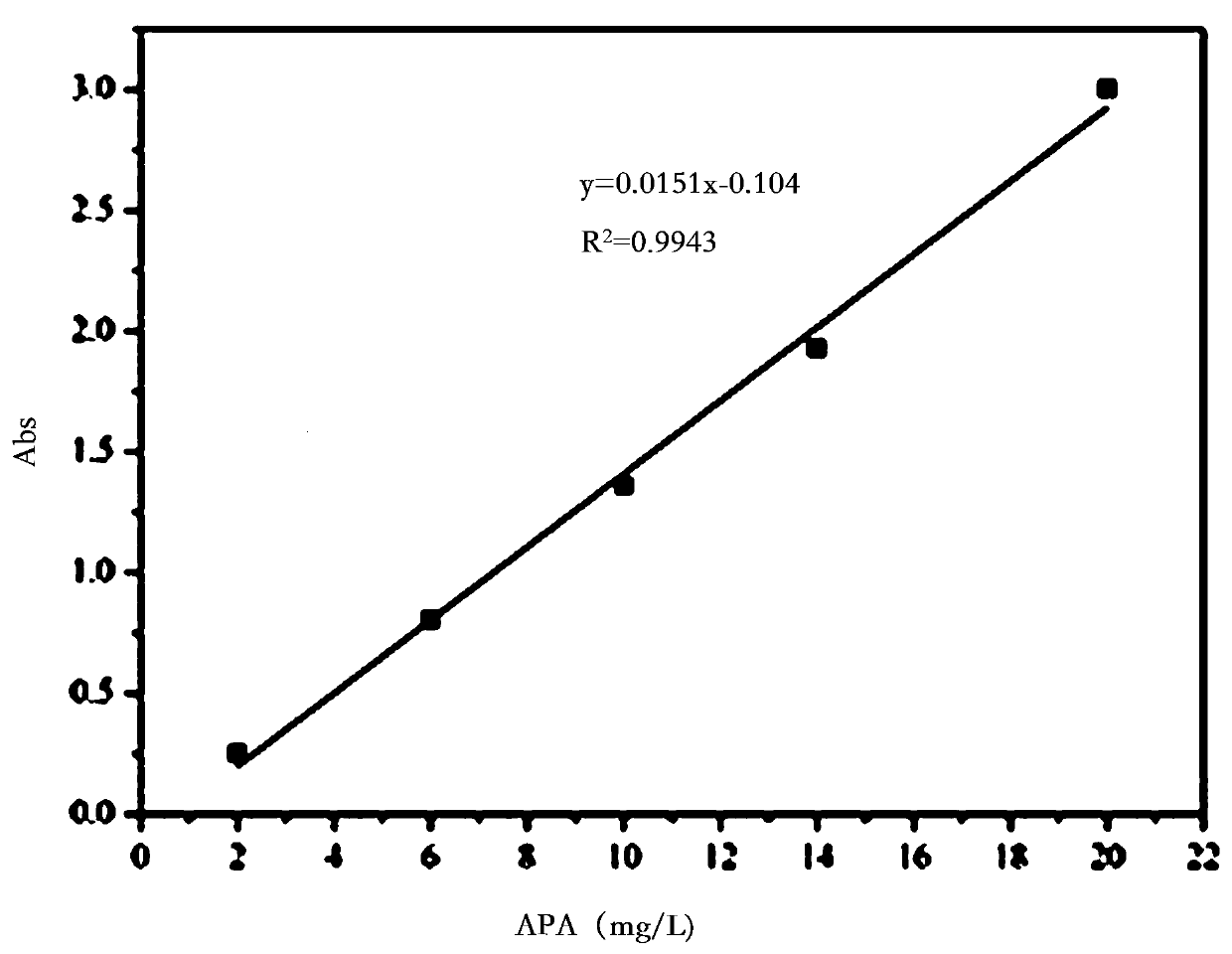
[0062] Drawing of the standard curve of apatinib aqueous solution: Weigh 5 mg of apatinib and dissolve it in 10 mL of ultrapure water, sonicate for 10 min and transfer it to a 25 mL volumetric flask after it is completely dissolved. Take 1, 3, 5, 7, and 10 mL of apatinib solution respectively, and dilute to volume in a 10 mL volumetric flask to prepare concentrations of 0.02, 0.06, 0.10, 0.14, and 0.20 mg / mL, respectively. Transfer 3.5mL of the prepared solution to the sample cell, and test its UV absorbance at a wavelength of 254nm, with blank ultrapure water as a reference.
[0063] according to image 3 It can be seen that the standard curve of apatinib: according to the absorbance corre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com