Amoxicillin biodegradable microsphere long-acting sustained-release preparation and preparation method thereof
A technology of amoxicillin and biodegradation, applied in the field of amoxicillin biodegradable microsphere long-acting sustained-release preparations and its preparation, can solve the problems of inability to maintain effective blood drug concentration, instability of β-lactam ring, short half-life, etc. problems, to achieve the effects of obvious sustained-release effect, no toxic side effects, and low burst release dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
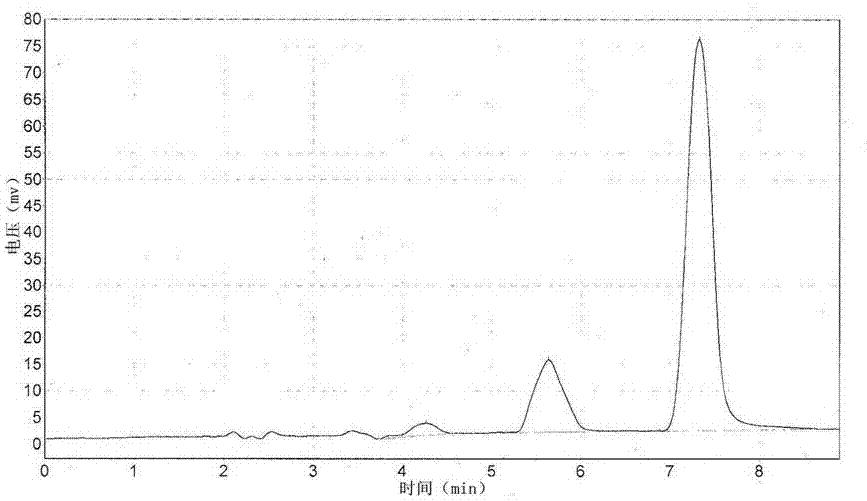
[0027] Long-acting sustained-release preparation of amoxicillin biodegradable microspheres with a drug concentration of 10%
[0028] The preparation method is as follows:
[0029] (1) Slowly add 1 g of PLGA into 10 ml of triacetin and heat to 50°C, stir slowly until it is completely dissolved to obtain a viscous liquid and let it stand at room temperature.
[0030] (2) Add 12g of amoxicillin raw material (95% amoxicillin content) into 10ml of polyethylene glycol 400, and add 1ml of Tween 80, stir rapidly to make it fully mixed.
[0031] (3) Slowly add the suspension solution obtained in (2) into the viscous liquid described in (1), and stir at high speed for 15-20 minutes, so that all the drugs are mixed evenly, and the paste-like semi-solid drug dispersion phase I is obtained at room temperature.
[0032] (4) Heat 70g of polyethylene glycol 4000 in a water bath to the molten state at 50°C as the continuous phase II, slowly add the drug dispersion phase I obtained in (3) into...
Embodiment 2
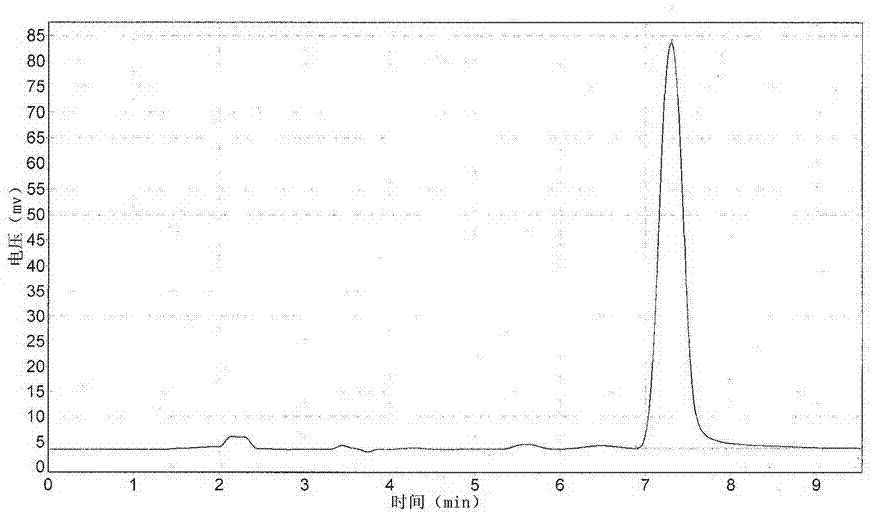
[0036] Long-acting sustained-release preparation of amoxicillin biodegradable microspheres with a drug concentration of 20%
[0037] The preparation method is as follows:
[0038] (1) Slowly add 2g of PLGA and 1g of PLA into 10ml of triacetin and heat to 50°C, stir slowly until they are completely dissolved to obtain a viscous liquid and let it stand at room temperature.
[0039] (2) Add 22g of amoxicillin raw material (95% amoxicillin content) into 12ml of polyethylene glycol 400, and add 1ml of Tween 80, stir rapidly to make it fully mixed.
[0040] (3) Slowly add the suspension solution obtained in (2) into the viscous liquid described in (1), and stir at high speed for 15-20 minutes, so that all the drugs are mixed evenly, and the paste-like semi-solid drug dispersion phase I is obtained at room temperature.
[0041] (4) Heat 50g of polyethylene glycol 6000 in a water bath to the molten state at 55°C as the continuous phase II, slowly add the drug dispersion phase I obtai...
Embodiment 3
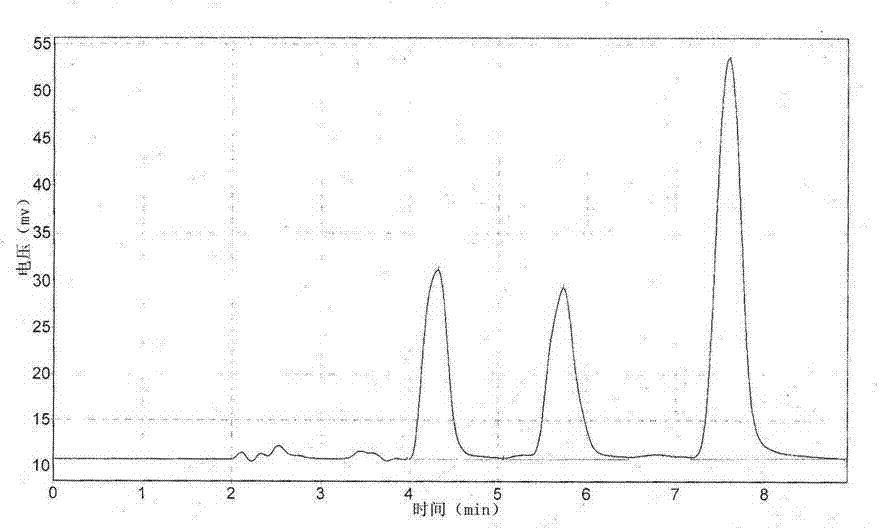
[0045] Long-acting sustained-release preparation of amoxicillin biodegradable microspheres with a drug concentration of 15%
[0046] The preparation method is as follows:
[0047] (1) Slowly add 1g of PLGA and 1g of PLA into 10ml of ethyl acetate, heat to 50°C, stir slowly until all are dissolved, obtain a viscous liquid and let it stand at room temperature.
[0048] (2) Add 18g of amoxicillin raw material (95% amoxicillin content) into 12ml of polyethylene glycol 400, and add 1ml of Tween 80, stir rapidly to make it fully mixed.
[0049] (3) Slowly add the suspension solution obtained in (2) into the viscous liquid described in (1), and stir at high speed for 15-20 minutes, so that all the drugs are mixed evenly, and the paste-like semi-solid drug dispersion phase I is obtained at room temperature.
[0050] (4) Heat 60g of polyethylene glycol 6000 in a water bath to the molten state at 60°C as the continuous phase II, slowly add the drug dispersion phase I obtained in (3) in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com