Preparation and application of HP-beta-CD (hydroxypropyl beta-cyclodextrin) functionalized GO (graphene oxide) composite material
A composite material, hydroxypropyl technology, applied in the field of electrochemical applications, composite material preparation, can solve the problems of no curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
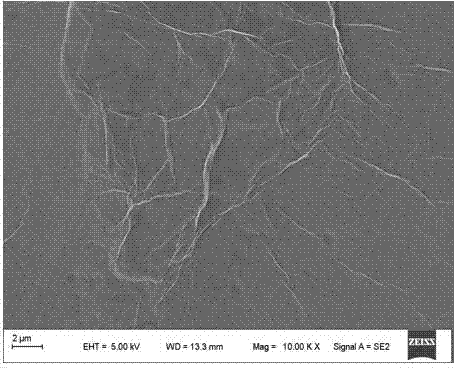
Image
Examples
Embodiment 1
[0033] Example 1. Preparation of rGO-HP-β-CD chiral composite material
[0034] (1) Preparation of hydroxypropyl β-cyclodextrin
[0035] First, add 1g of β-CD to KOH (20wt%) solution, stir and react at room temperature for 6 hours, in order to fully activate β-CD, put it in the refrigerator for 22 hours, then add 3ml of propylene oxide, and stir at room temperature for 40~ 48 hours. Then use 10mM / L hydrochloric acid to adjust the pH of the mixed solution to 7, rotary steam at 45~50°C, then dissolve the product with 20ml of ethanol, filter with suction, add 200ml of acetone to the filtrate, stir quickly, filter with suction, wash, And vacuum-dried at 50-60°C for 60-72 hours to obtain white HP-β-CD powder, HP-β-CD has better water solubility than β-CD.
[0036] (2) Preparation of graphene oxide (GO)
[0037] Using the modified Hummers method, 1.5g flake graphite was placed in 20ml concentrated sulfuric acid dissolved with 2.5g potassium persulfate and 2.5g phosphorus pentoxid...
Embodiment 2
[0040] Embodiment 2, the preparation of chiral electrochemical sensor
[0041] The bare glassy carbon electrode (GCE) was coated with 0.3 μm, 0.05 μm and 0.02 μm Al on the suede 2 o 3 For polishing, first rinse the surface dirt with ultrapure water, then transfer to ethanol and ultrapure water for ultrasonication for 5~6min respectively. 5 mL of rGO-HP-β-CD with a mass concentration of 30 μg / ml was uniformly drop-coated on the surface-polished GCE to prepare a rGO-HP-β-CD / GCE chiral electrochemical sensor, and then rGO-HP-β- CD / GCE were placed in 5.0~6.0mM [Fe(CN) 6 ] 4- / 3- The solution (the solution contains 0.1~0.15M KCl) was tested by cyclic voltammetry (CV), and the peak current of the CV curve was 0.033mA.
Embodiment 3
[0042] Example 3. Recognition of Tryptophan Enantiomers by Chiral Electrochemical Sensors
[0043] The chiral electrochemical sensor rGO-HP-β-CD / GCE was respectively placed in the L-tryptophan or D-tryptophan solution with a concentration of 8-10mM / L and a volume of 20-25mL (the solution contained 0.2 ~0.3M PBS (pH=6.0) buffer solution and 0.5mM [Fe(CN) 6 ] 4- / 3- ), using differential pulse voltammetry (DPV) for chiral recognition of tryptophan enantiomers, scanning potential from 0.3 to 1.4V, scanning speed 100mV s -1 . When L-tryptophan and D-tryptophan interact with rGO-HP-β-CD / GCE, the peak current values are different, so that the purpose of identifying the enantiomers of tryptophan can be achieved. When rGO-HP-β-CD / GCE interacted with L-tryptophan, the peak current was D-tryptophan.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com