Immobilized beta-glucosaccharase and preparation method and application thereof
A glucosidase and reaction technology, applied in the fields of enzyme engineering and bioengineering applications, can solve the problem of high use cost, and achieve the effects of improving catalytic efficiency, improving pH stability, and improving glucose tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The β-glucosidase clone and recombinant enzyme preparation of the GH3 family of embodiment 1.T.petrophila DSM 13995 origin
[0032] 1.1 Culture of T.petrophila DSM 13995
[0033] Thermotoga petrophila DSM 13995 was purchased from the DSMZ Culture Collection Center (www.dsmz.de) with the number 13995. The medium formula is: 10g / L starch, 5g / L tryptone, 3g / L yeast extract, 5g / L meat extract, 10g / L2‐morpholinoethanesulfonic, 10mg / L FeSO 4 ×7H 2 O, 1mg / Lresazurin, adjust the pH to 7.2. Inoculate with a syringe according to 0.5% inoculation amount, culture statically at 85° C. for 24 hours, and collect cells.
[0034] 1.2 Genomic DNA extraction
[0035] (1) Statically culture Thermotoga petrophila DSM 13995 for about 24 hours, take 30 mL of bacterial liquid and centrifuge at 4,000 g for 10 min to collect cells.
[0036] (2) Resuspend the bacteria in 9.5 mL TE buffer, add 0.5 mL 10% sodium dodecyl sulfate (SDS) and 50 μL proteinase K (20 mg / mL), mix well, and incubate at...
Embodiment 2
[0050] Example 2. Preparation of immobilized enzyme (NKA-9II) by immobilizing β-glucosidase derived from T.petrophila DSM 13995GH3 family using macroporous adsorption resin
[0051] 2.1 Macroporous resin pretreatment
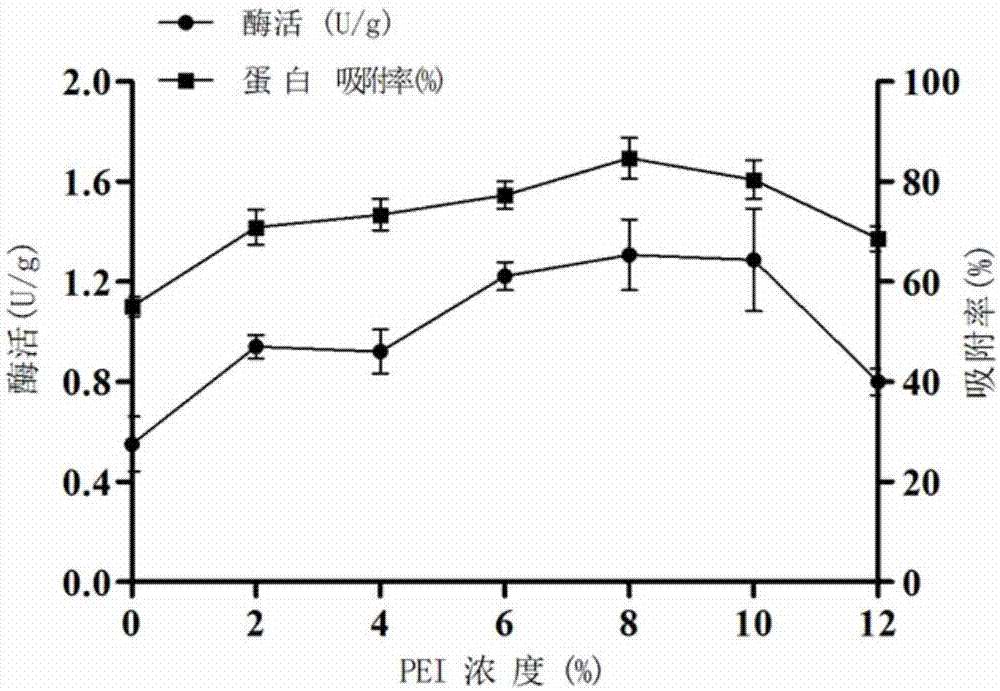
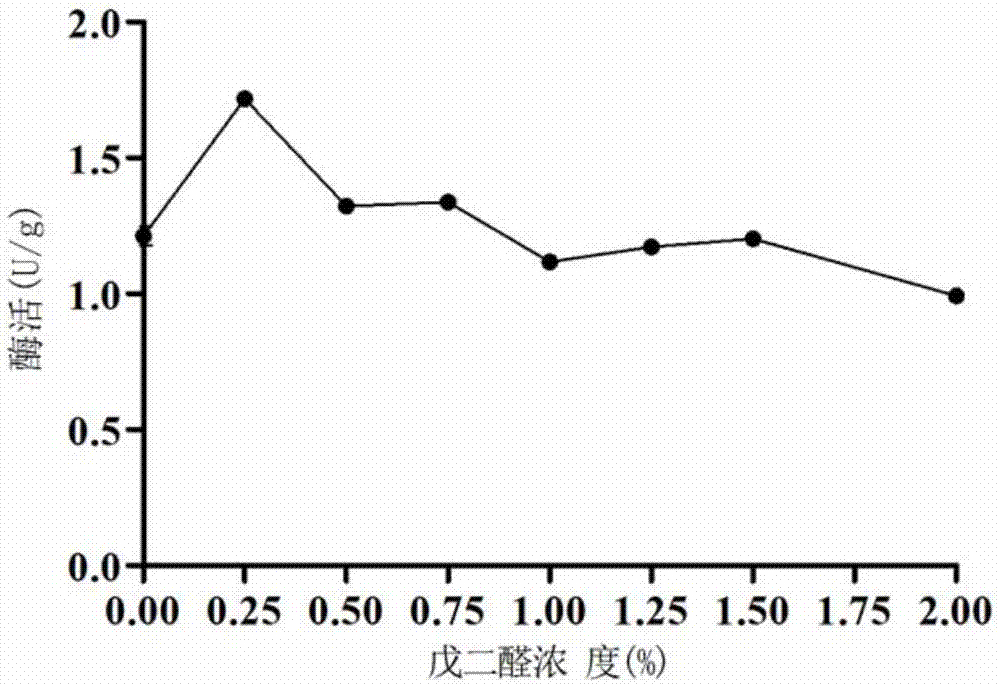
[0052] In order to remove impurities and pollutants in the macroporous resin, and more effectively maximize the amount of enzyme protein adsorbed by the macroporous adsorption resin carrier, the experiment pretreated the macroporous resin by adding ethanol, hydrochloric acid and sodium hydroxide respectively. First, the macroporous resin (20 g) was added to 100 mL of absolute ethanol and soaked for 24 hours, and then washed with deionized water repeatedly for 3 times until no ethanol smell remained. Then the treated macroporous resin was soaked in 100 mL solution containing 5% hydrochloric acid for 4 h, and then the resin was washed to neutral pH with deionized water. Then soak the cleaned resin in a solution containing 1M sodium hydroxide (100 mL) for 4 hours,...
Embodiment 3
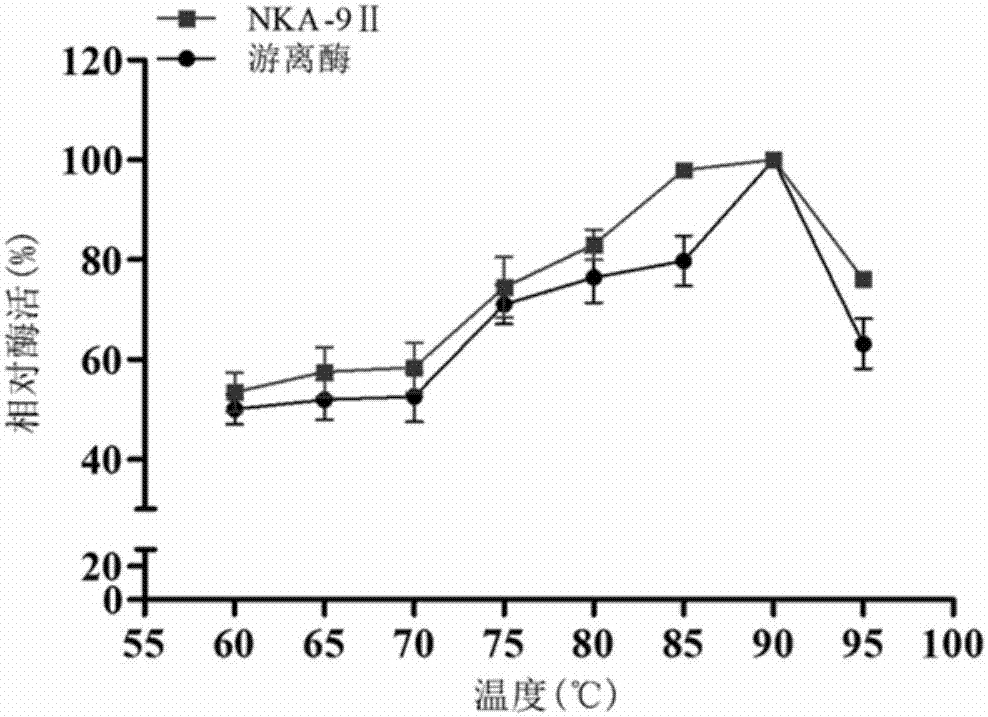
[0097] Example 3. Zn 2+ Effects on the enzymatic properties of the immobilized enzyme NKA‐9II
[0098] 3.1. Metal cation Zn 2+ Effect on the catalytic activity of the prepared immobilized enzyme NKA-9II
[0099] Experiment by comparing different metal cations Fe 2+ , Li 2+ , Al 3+ , K + , Ni 2+ , Mn 2+ , Ca 2+ , Zn 2+ and Mg 2+ The impact on the catalytic activity of the immobilized enzyme NKA-9II, the results are shown in Table 2, the metal ion Mn 2+ and Zn 2+ The catalytic efficiency of the immobilized enzyme can be significantly improved. mn 2+ It can increase the enzyme activity of immobilized enzyme by 45%; Zn 2+ It can increase the enzyme activity of immobilized enzyme by 92%, the results show that Zn 2+ effect is more obvious.
[0100] 3.2. Zn 2+ Effect on the reproducibility of the prepared immobilized enzyme NKA-9II
[0101] In the presence or absence of 2mM Zn 2+ In the case of participation, 10 repeated reactions were carried out, the results are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com