Efficient IL-2 (interleukin-2) mutant fusion protein and application thereof
An interleukin and fusion protein technology, applied in the field of human interleukin II mutant fusion protein, can solve the problems of application and curative effect limitations, short half-life of interleukin II, etc., to eliminate toxic and side effects, improve clinical efficacy and application scope, Enhance the effect of attributes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Construction of Human Interleukin II Mutant Library
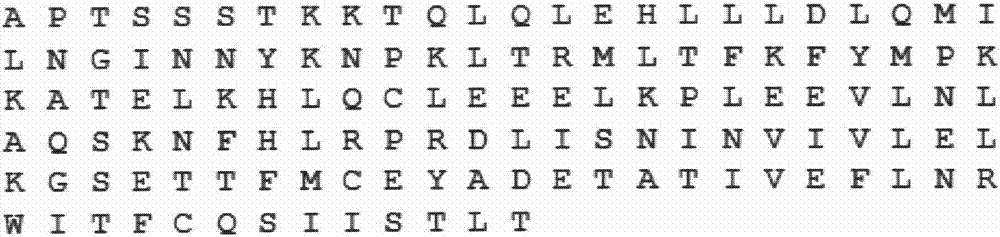
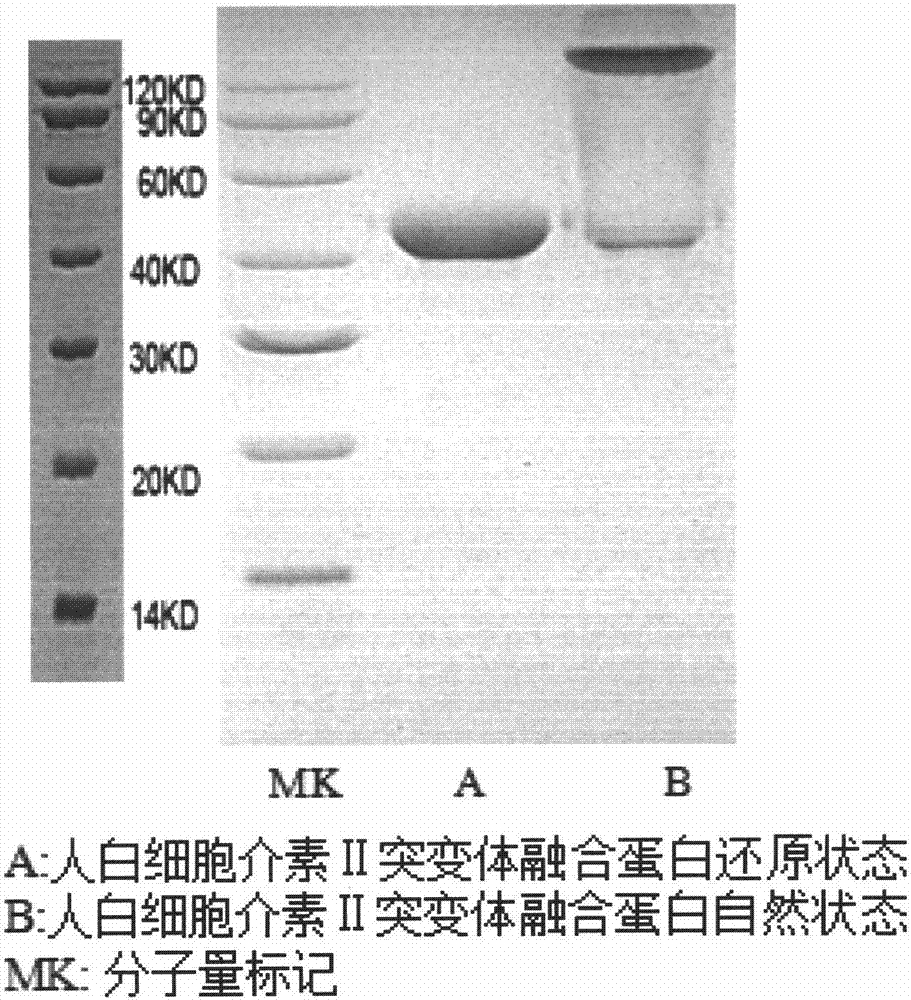
[0020] According to the amino acid sequence of human interleukin II (gene bank NM-000586) ( figure 1 ) to mutate the gene sequence. Synthesis of human interleukin II mutant fusion protein gene by overlap extension method ( figure 2 ), and further cloned into a multifunctional expression vector. These vectors were transfected into Chinese hamster ovary cells, and the cells were placed at 37°C, 5% CO 2 After culturing in an incubator, the supernatant was taken after 72 hours, and further purified into an interleukin II mutant fusion protein by immobilized metal ion affinity chromatography ( image 3 ). The purified protein was detected by electrophoresis to confirm the molecular weight, and was further used in the study of immune cell proliferation in vivo.
Embodiment 2
[0022] Mutant interleukin II to promote suppressor (CD4CD25+) T cells, NK cells and CD8+ T cell activity assay
[0023] Black mice were injected intraperitoneally with interleukin II protozoa or interleukin II mutant fusion protein. The daily dose of protozoa was 20 micrograms for a total of 4 days, and the interleukin II mutant fusion protein was 4 micrograms for a total of 4 days. The spleen was collected on the fifth day. Splenocytes were stained using CD25, CD8, NK1.1 and CD4 antibody staining. Finally, flow cytometry was used to measure and data analysis. Figure 4 and 5 showed that compared with protozoa, interleukin II mutant fusion protein enhanced the function of NK (from 14.7% to 26.8%) and CD8 (from 14.9% to 23.7%) cell expansion, while attenuating the effect on CD4+CD25+ Expansion of regulatory cells (from 4.11% to 0.97%).
Embodiment 3
[0025] Effects of Mutant Interleukin II Fusion Protein and Prototype Interleukin II Protein on the Ratio of Effector and Inhibitor Cells
[0026] as attached Figure 6 , as shown in 7, mice were injected intraperitoneally with interleukin II protobody or interleukin II mutant fusion protein, compared with the original body, the interleukin II mutant fusion protein increased the ratio of NK cells to regulatory suppressor cells (from 3.8 to 24.1)( Figure 7 ), increased the ratio of CD8 to regulatory suppressor cells (from 4.0 to 23.8) ( Figure 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com