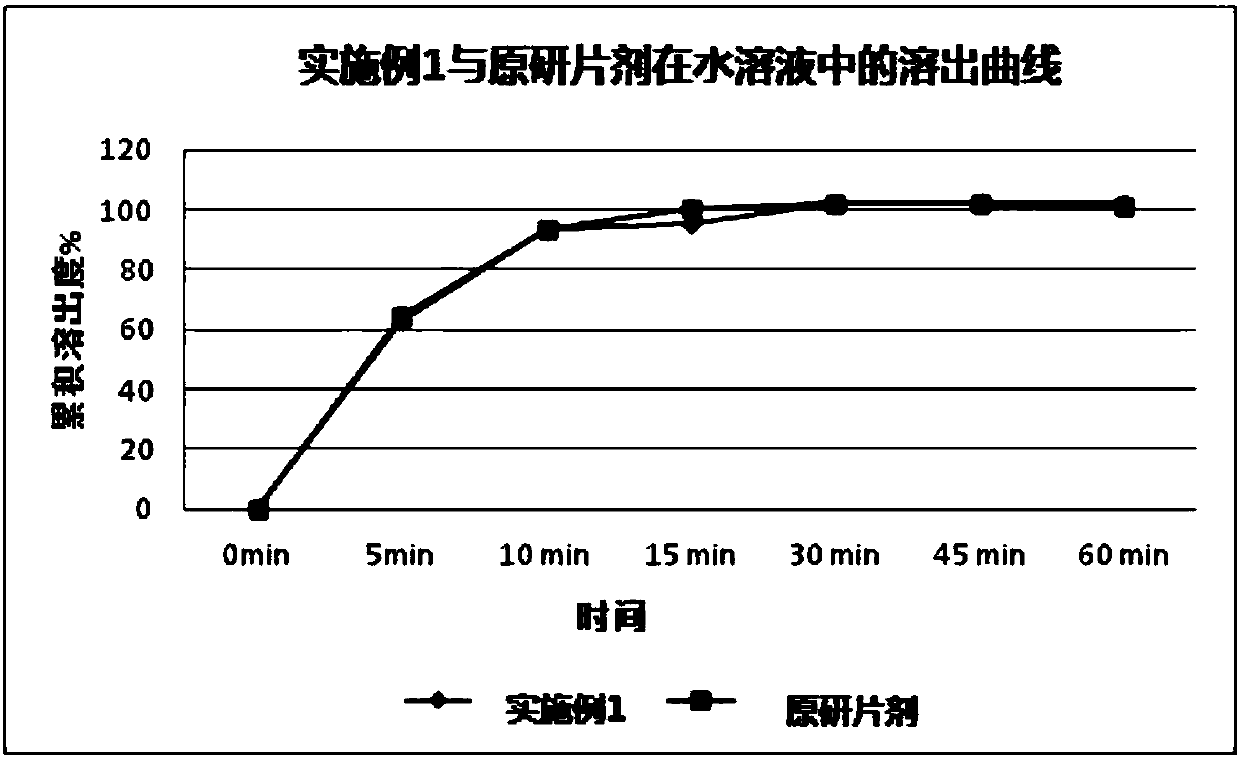
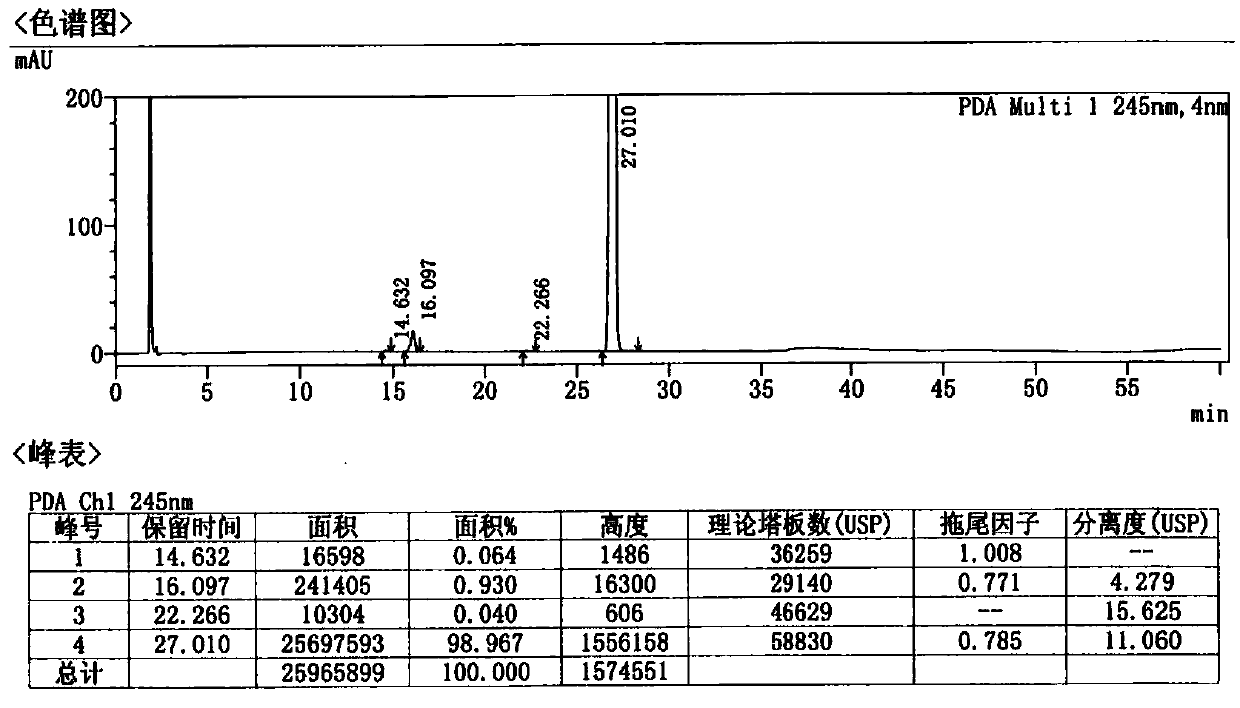
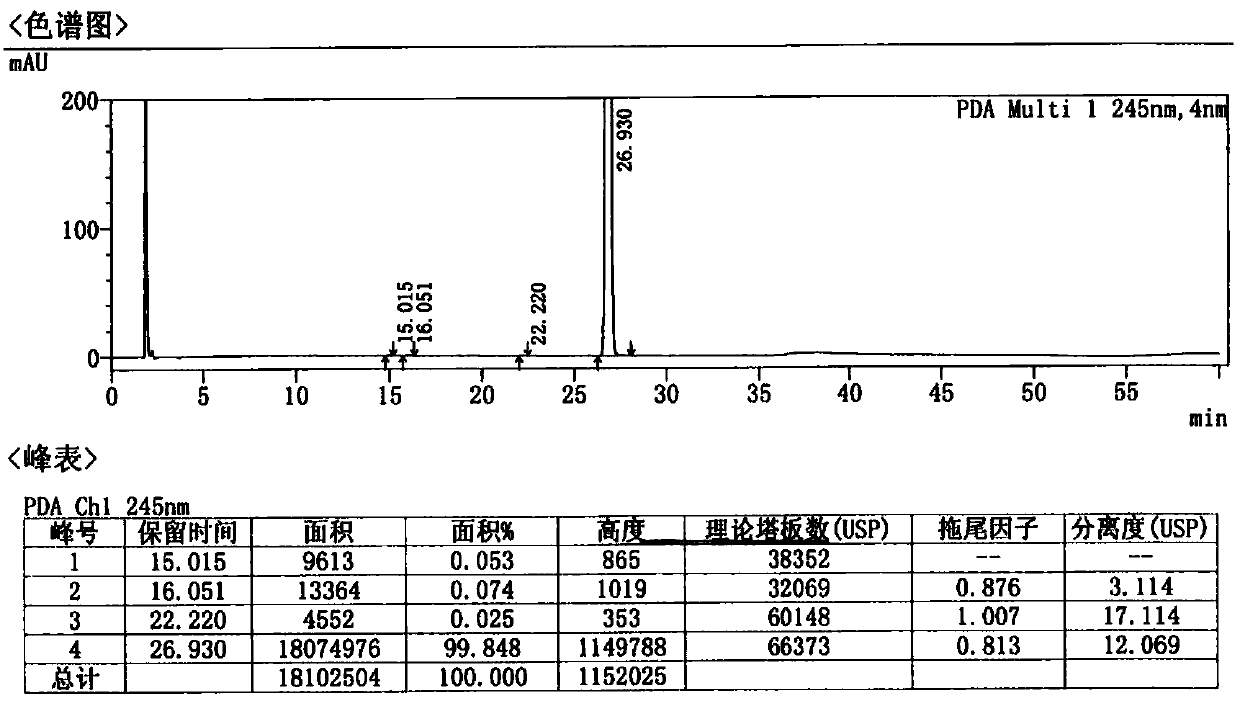
A kind of preparation method of Afatinib maleate tablet
A technology of maleic acid and alpha, which is applied in the field of preparation of maleic acid afatinib tablets to achieve the effects of improving stability, avoiding drying, and uniform content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3 and comparative example 1-10
[0041] The preparation of embodiment 1-3 and comparative example 1-10 Afatinib maleate sheet
Embodiment 1-3
[0042] Example 1-3 The composition of Afatinib Maleate Tablets is shown in the table below:
[0043]
[0044]
Embodiment 1
[0049] Example 1 Preparation of Afatinib Maleate Tablets:
[0050] (1) In parts by weight, take respectively afatinib maleate 29.56g, mannitol 118g, microcrystalline cellulose PH10124g, polyethylene glycol PEG6000 30g, calcium sulfate 10g, crospovidone 3.6g, Magnesium stearate 0.5g, silicon dioxide 2g, stomach-soluble film coating premix ( 295B680002) 5.4g, spare;
[0051] (2) Mix afatinib maleate, mannitol, microcrystalline cellulose PH101, polyethylene glycol PEG6000 and calcium sulfate (2.5g) evenly, heat to 70°C, keep warm for 15 minutes after reaching the predetermined temperature, Cool at room temperature, add crospovidone, extra calcium sulfate (7.5g), magnesium stearate, and silicon dioxide after granulation, mix evenly, and compress into tablets;
[0052] (3) Use the gastric-soluble film coating premix ( 295B680002) was film-coated to obtain Afatinib maleate tablets.
[0053] Examples 2 and 3 The preparation of afatinib maleate tablets is shown in reference ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com