Method for preparing levofloxacin hydrochloride
A technology of levofloxacin hydrochloride and cyclic acid, which is applied in the field of organic compound synthesis, can solve the problems of increased post-processing workload and three wastes, poor environmental protection and economic efficiency, and no specific introduction, so as to reduce environmental pollution and consumption of industrial raw materials, and reduce production costs , The effect of simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
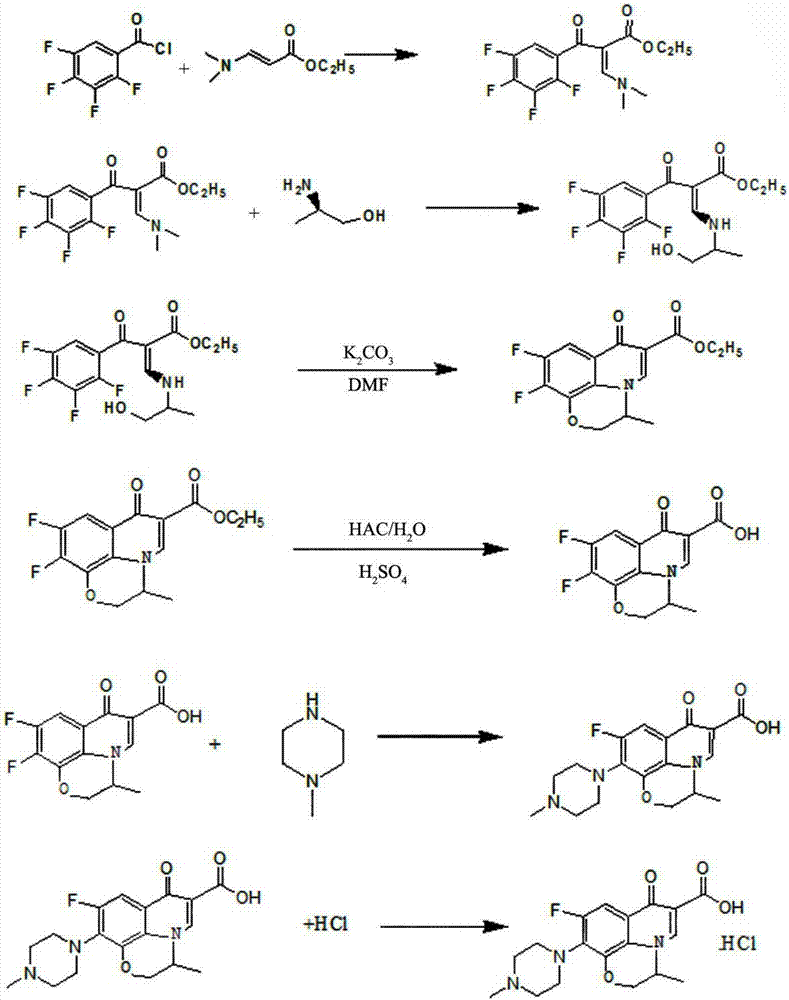
[0044] Such as figure 1 The synthetic method of shown levofloxacin hydrochloride comprises the following steps:
[0045] (1) Preparation of 3-(2-hydroxy-1-methyl-ethylamino)-2-(2,3,4,5-tetrafluorobenzoyl)-ethyl acrylate
[0046]Add 29.2g of N,N ethyl dimethylaminoacrylate, 22.7g of triethylamine and 340ml of toluene into a three-necked flask, heat to 50°C under stirring, slowly add a solution of 42.5g of tetrafluorobenzoyl chloride / toluene, dropwise Add for 1.5 hours, keep warm for 3 hours after dropping, convert 97%, cool, filter about 24.2g of triethylamine hydrochloride, wash the filter cake of triethylamine hydrochloride with a small amount of toluene, heat the filtrate to 50°C, add 15g of triethylamine hydrochloride dropwise L-2-Aminopropanol, dripped in half an hour, raised the temperature to 90°C, kept it warm for 1 hour, the conversion was 98%, cooled, washed twice with water, the toluene phase was separated, spin-dried and dehydrated, diluted with DMF to 250ml, liqui...
Embodiment 2
[0053] Such as figure 1 The synthetic method of shown levofloxacin hydrochloride comprises the following steps:
[0054] (1) Preparation of 3-(2-hydroxy-1-methyl-ethylamino)-2-(2,3,4,5-tetrafluorobenzoyl)-ethyl acrylate
[0055] Add 29.2g of N,N ethyl dimethylaminoacrylate, 20.2g of triethylamine and 340ml of toluene into a three-necked flask, heat to 55°C under stirring, slowly add a solution of 46.8g of tetrafluorobenzoyl chloride / toluene, dropwise Add for 1 hour, keep warm for 2 hours after dropping, convert 96%, cool, filter about 24.8g of triethylamine hydrochloride, wash the filter cake of triethylamine hydrochloride with a small amount of toluene, heat the filtrate to 40°C, add 16.5g of it dropwise The L-2-aminopropanol was dropped in half an hour, heated to 90°C, kept warm for 0.5 hours, converted 98%, cooled, washed three times with water, separated into toluene phases, spin-dried and dehydrated, diluted with DMF to 250ml, liquid The phase purity is 99.22%.
[0056...
Embodiment 3
[0062] Such as figure 1 The synthetic method of shown levofloxacin hydrochloride comprises the following steps:
[0063] (1) Preparation of 3-(2-hydroxy-1-methyl-ethylamino)-2-(2,3,4,5-tetrafluorobenzoyl)-ethyl acrylate
[0064] Add 29.2g of N,N ethyl dimethylaminoacrylate, 24.3g of triethylamine and 340ml of toluene into a three-necked flask, heat to 60°C under stirring, slowly add 45g of tetrafluorobenzoyl chloride / toluene solution dropwise, dropwise 2 hours, keep warm for 4 hours after dripping, conversion 97%, cooling, filter about 25.2g of triethylamine hydrochloride, wash the filter cake of triethylamine hydrochloride with a small amount of toluene, heat up the filtrate to 60°C, add dropwise 16g of L -2-Aminopropanol, drop it in half an hour, raise the temperature to 90°C, keep it warm for 1.5 hours, the conversion is 98%, cool, wash twice with water, separate the toluene phase, spin dry and dehydrate, add DMF to dilute to 250ml, liquid phase The purity is 99.41%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com