A kind of repair material with high induction activity, preparation method and application
A technology for repairing materials and inducing activity, which can be applied in prosthetics, tissue regeneration, medical science, etc. It can solve the problems of short half-life of growth factors and achieve the effect of long action period and controllable degradation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation method of the animal-derived implantable biological patch in this embodiment includes the following steps:
[0051] (1) Organization pre-processing:
[0052] Take the small intestinal submucosa tissue material and divide it into the specified size of 10cm in width and 15cm in length, remove the lymphoid tissue, wash it with tap water for 3 times, and then rinse it with purified water until there is no stain on the surface, and place the washed small intestinal submucosa tissue material on the filter Wait for the water filter device, let it stand for more than 5 minutes, and drain the water.
[0053] (2) Virus inactivation:
[0054] Use peracetic acid-ethanol solution to soak small intestinal submucosa tissue material for virus inactivation, and this process can be carried out in a stainless steel bucket. The volume percent concentration of peracetic acid in the peracetic acid-ethanol solution is 2%, the volume percent concentration of ethanol is 20%, a...
Embodiment 2
[0072] Example 2: Performance testing
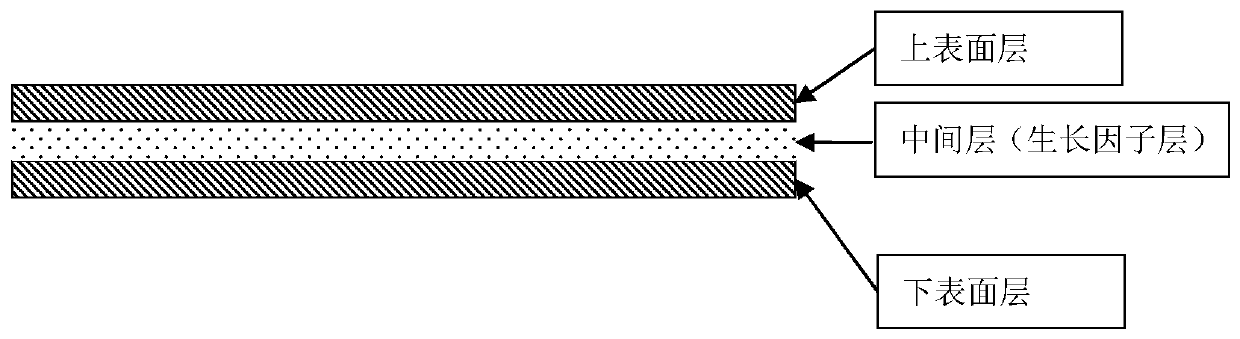
[0073] 1) The heterogeneous immunogen removal matrix material with high inductive activity is a three-layer structure, the upper and lower surface layers are complete surface layers, and the middle layer is a layer containing growth factors, forming a sandwich structure. The surface layer is relatively dense, which can control the degradation behavior of the material and growth factor release speed, and the growth factor layer is a porous structure layer, which is beneficial to preserve the growth factor. The porosity of the surface layer is 67.4±5.2%, while that of the growth factor layer is 90.5±7.0%.
[0074] 2) The test of mechanical properties shows that the tensile breaking strength of the sample reaches 105N / cm, and the suture holding force is greater than 21N.
[0075] 3) According to the fourth part of the biological preparation residual DNA detection method "Chinese Pharmacopoeia" 2015 edition, the residual DNA of the sample p...
Embodiment 3
[0081] Embodiment 3: the detection of active factor release curve
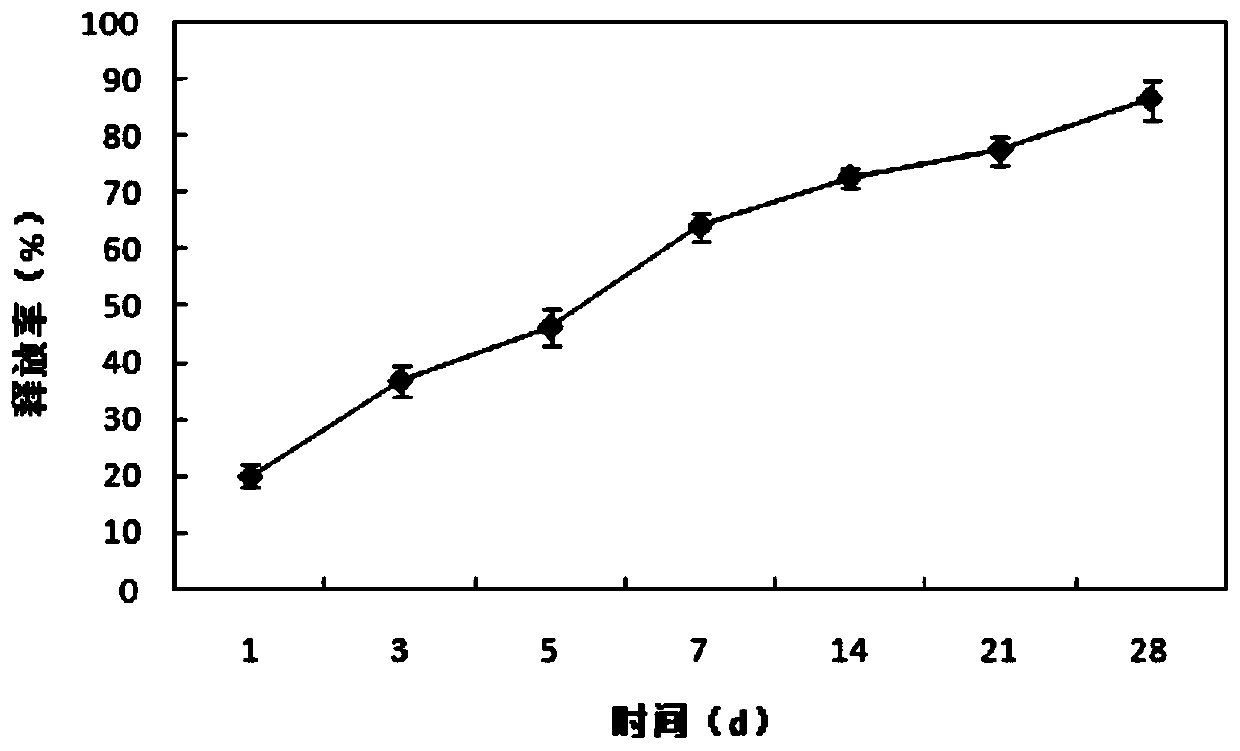
[0082]Removal of heterogeneous immunogens with highly inducible activity carrier proteins The matrix material was prepared according to Example 1. The samples were placed in a 12-well plate, and 2 ml of PBS (pH 7.4) was added to each well. 5% CO at 37°C with saturated humidity 2 Atmosphere CO 2 Incubate in the incubator for 1, 3, 5, 7, 14, 21, 28 days (days), then transfer the PBS to a centrifuge tube, and detect the content of platelet-derived growth factor M1 in the PBS solution, and add the initial addition amount as the denominator M0 , Calculate growth factor release rate = M1 / M0. as attached image 3 As shown, the release of growth factors was stable and controllable. The growth factor release rate was 19.92±1.96% on the first day, 46.32±3.36% on the third day, 64.16±2.50% on the seventh day, and the total release rate on the 28th day The rate reaches 86.56±3.42%. Therefore, the growth factor can la...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com