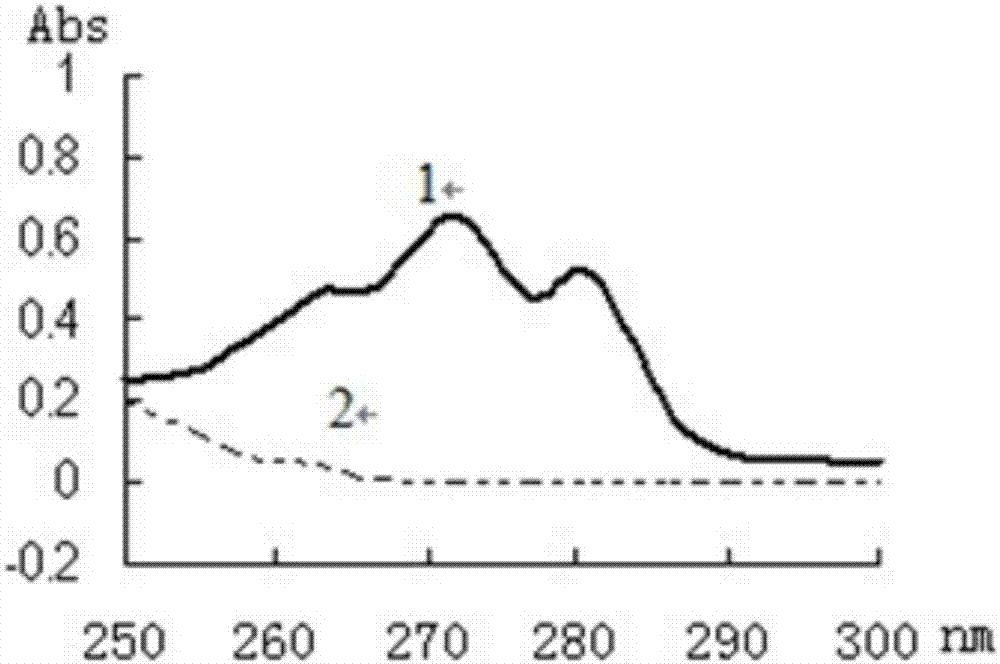
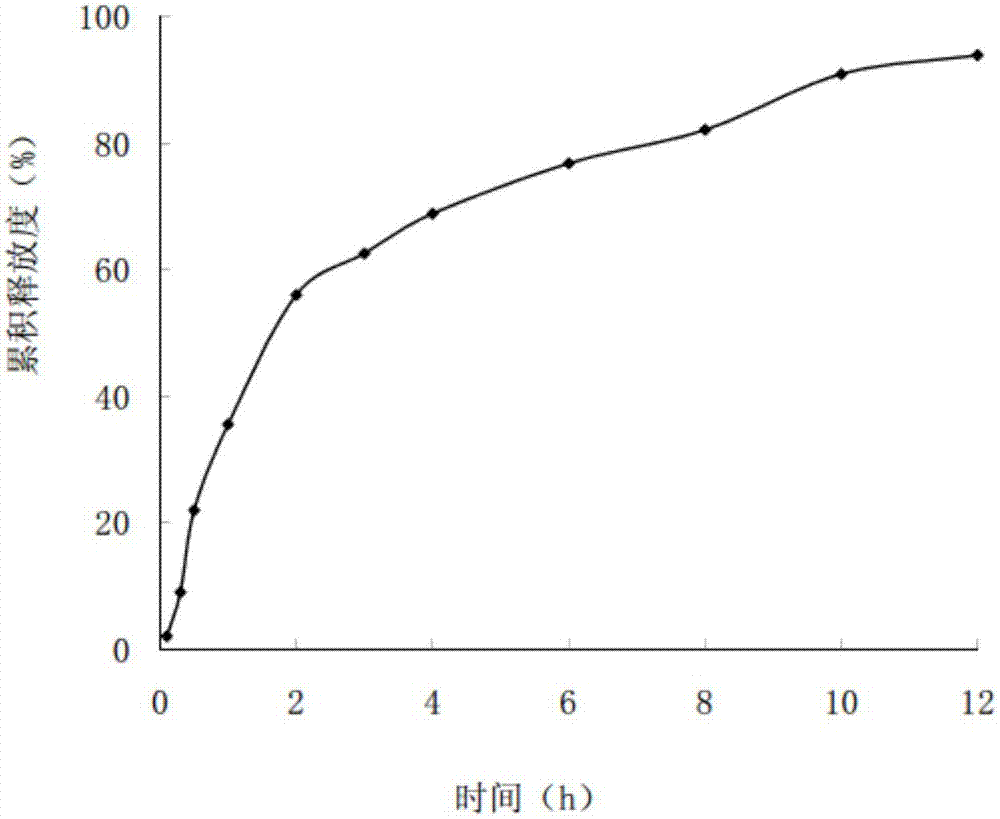
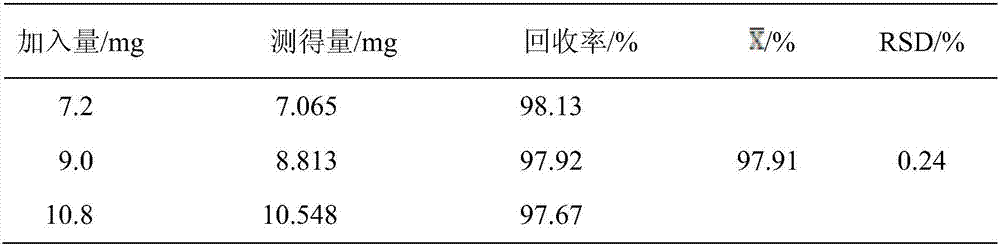
Miconazole nitrate-chitosan sustained-release vaginal gel and preparation method thereof
A technology of miconazole nitrate and chitosan nitrate, which is applied in the field of medicine, can solve the problems that it is difficult to achieve the effect, the suppository has a large drug load, and is easy to produce drug resistance, so as to prolong the drug retention time and ensure the curative effect. , the effect of reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 A kind of miconazole nitrate chitosan vaginal sustained-release gel
[0025] The sustained-release gel consists of the following components in parts by weight:
[0026] 2 parts of miconazole nitrate, 0.5 parts of chitosan, 1 part of polycarbophil, 5 parts of glycerin, 2 parts of glyceryl behenate, 0.3 parts of sodium benzoate, 0.2 parts of triethanolamine, 2% acetic acid solution 15 parts, add water for injection to 100 parts.
[0027] The method for preparing miconazole nitrate chitosan vaginal sustained-release gel, the concrete steps are as follows:
[0028] (1) Prepare chitosan acetic acid solution: take chitosan, add in the beaker that fills the acetic acid solution of 2% by mass fraction, stir until dissolving;
[0029] (2) Preparation of polymer phase: Soak polycarbophil with an appropriate amount of water for injection overnight, fully stir until fully swollen into a uniform gel-like polymer, slowly add the chitosan acetate solution prepared in st...
Embodiment 2
[0033] Embodiment 2 A kind of miconazole nitrate chitosan vaginal sustained-release gel
[0034] The sustained-release gel consists of the following components in parts by weight:
[0035] 1.2 parts of miconazole nitrate, 0.3 parts of chitosan, 0.7 parts of polycarbophil, 4.5 parts of glycerin, 1.3 parts of glyceryl behenate, 0.3 parts of sodium benzoate, 0.15 parts of triethanolamine, and an acetic acid solution with a mass fraction of 2%. 13 parts, add water for injection to 100 parts.
[0036] The method for preparing miconazole nitrate chitosan vaginal sustained-release gel, the concrete steps are as follows:
[0037] (1) Prepare chitosan acetic acid solution: take chitosan, add in the beaker that fills the acetic acid solution of 2% by mass fraction, stir until dissolving;
[0038] (2) Preparation of polymer phase: Soak polycarbophil with an appropriate amount of water for injection overnight, fully stir until fully swollen into a uniform gel-like polymer, slowly add th...
Embodiment 3
[0042] Example 3 A miconazole nitrate chitosan vaginal sustained-release gel
[0043] The sustained-release gel consists of the following components in parts by weight:
[0044] 3 parts of miconazole nitrate, 0.7 parts of chitosan, 1.5 parts of polycarbophil, 6.5 parts of glycerin, 2.8 parts of glyceryl behenate, 0.5 parts of sodium benzoate, 0.3 parts of triethanolamine, and an acetic acid solution with a mass fraction of 2%. 16 parts, add water for injection to 100 parts.
[0045] The method for preparing miconazole nitrate chitosan vaginal sustained-release gel, the concrete steps are as follows:
[0046] (1) Prepare chitosan acetic acid solution: take chitosan, add in the beaker that fills the acetic acid solution of 2% by mass fraction, stir until dissolving;
[0047] (2) Preparation of polymer phase: Soak polycarbophil with an appropriate amount of water for injection overnight, fully stir until fully swollen into a uniform gel-like polymer, slowly add the chitosan ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com