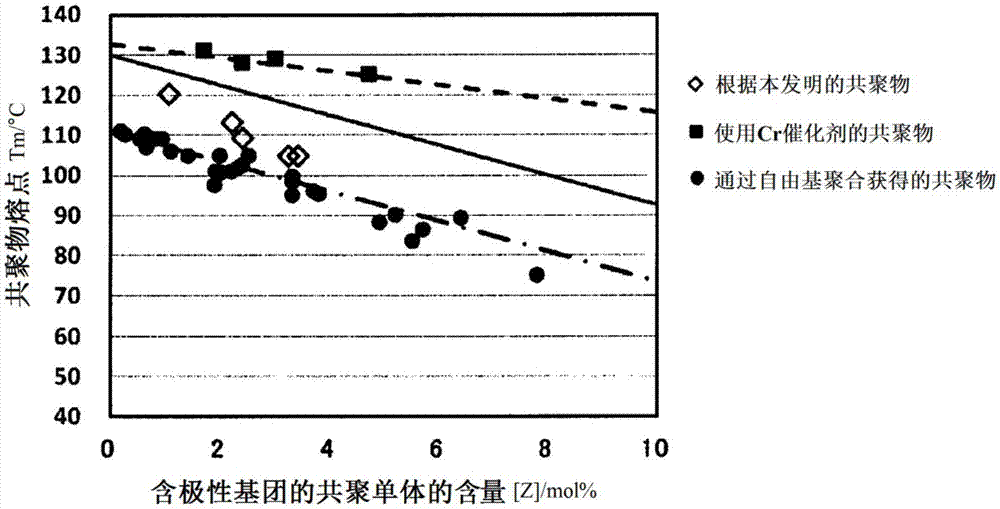
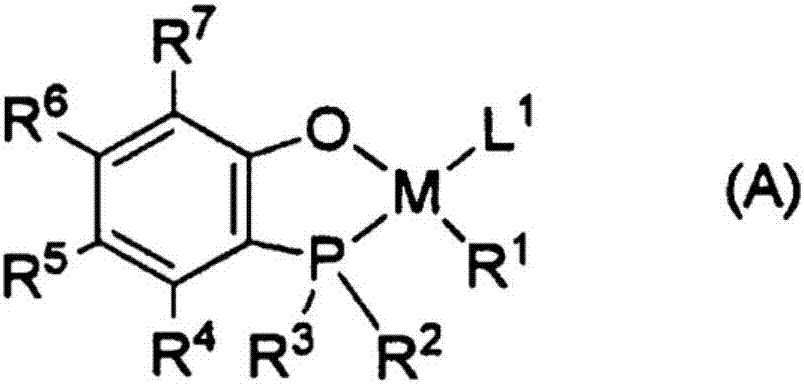
Production method for ethylene-based ionomer and ethylene-based ionomer
A manufacturing method and ionomer technology, which are used in the manufacture of ethylene ionomers and the fields of ethylene ionomers, can solve the problems of inability to obtain olefin copolymers and low polymerization activity, and achieve excellent elongation at break, excellent The effect of tensile properties and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0304] Hereinafter, the present invention will be described in more detail with reference to Examples and Comparative Examples, but the present invention is not limited to these Examples.
[0305] Measurement and evaluation of physical properties in Examples and Comparative Examples were performed by the following methods.
[0306]
[0307] (1) Measurement of the phase angle δ (G*=0.1MPa) when the absolute value of the composite elastic modulus G*=0.1MPa
[0308] 1) Pretreatment of samples
[0309] In the case where an acid group is included in the sample, for example, esterification treatment such as methylation using diazomethane, tetramethylsilane (TMS) diazomethane or the like is performed, and the resulting product is used for measurement.
[0310] 2) Sample preparation and measurement
[0311] After placing the sample in a mold for a heating press with a thickness of 1.0mm and preheating for 5 minutes in a heating press with a surface temperature of 180°C, the residu...
manufacture example 1
[0363] Production Example 1: Ethylene / tert-butyl acrylate copolymer (EtBA)
[0364] ·Synthesis of metal complexes
[0365] As the metal complex, according to Synthesis Example 4 described in the pamphlet of International Publication No. 2010 / 050256, a ligand B-27DM represented by the following chemical formula was used. According to Example 1 of the pamphlet of International Publication No. 2010 / 050256, bis-1,5-cyclooctadiene nickel(0) (referred to as Ni(COD) 2 ), synthesized B-27DM and Ni(COD) 2 Nickel complexes that have been reacted in a 1:1 ratio.
[0366]
[0367] Copolymerization of ethylene and tert-butyl acrylate (tBA)
[0368] Dry toluene (1.0 L), 36.6 mg (0.1 mmol) of tri-n-octylaluminum (TNOA) and 14.6 ml (100 mmol) of tert-butyl acrylate were placed in an autoclave with an inner volume of 2.4 L equipped with stirring blades. While stirring, the autoclave was heated to 90° C., nitrogen was supplied thereto at up to 0.3 MPa, ethylene was supplied to the autocl...
manufacture example 2
[0369] Production Example 2: Ethylene / tert-butyl acrylate copolymer (EtBA)
[0370] ·Synthesis of metal complexes
[0371] As the metal complex, according to Synthesis Example 10 described in the pamphlet of International Publication No. 2010 / 058849, a ligand represented by the following chemical formula (I) was used. According to Example 1-1 of the pamphlet of International Publication No. 2010 / 058849, bisdibenzylideneacetone palladium (referred to as Pd(dba) 2 ), where I and Pd(dba) were synthesized 2 Palladium that has been reacted in a 1:1 ratio.
[0372]
[0373] Copolymerization of ethylene and tert-butyl acrylate (tBA)
[0374] After an autoclave equipped with stirring blades having an inner volume of 2.4 liters was purged with purified nitrogen, dry toluene (0.8 liters) and 233 ml (1.6 mol) of tert-butyl ester (tBA) were charged thereinto. While stirring, the autoclave was heated to 110° C., nitrogen was supplied thereto up to 0.3 MPa, and ethylene was supplied ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com