In vitro telomere extension and proliferation culture method of chondrocytes and human tissue engineered regeneration of cartilage
A chondrocyte and telomere lengthening technology, applied in the field of cell biology, can solve the problems of inability to walk, affect the quality of life, pain, etc., achieve the improvement of safety and applicability, increase the activity of telomerase, and prolong the length of telomeres. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Telomerase mRNA and complete medium to treat chondrocytes from elderly patients
[0050] 1. Telomerase mRNA must first be synthesized in vitro. Plasmid pBABE-neo-hTERT (plasmid 1774, Addgene, Cambridge, MA, USA) was amplified by PCR (primers were SEQ ID No.2 and SEQ ID No.3), and the TERT CDS region containing telomerase was amplified DNA (SEQ ID No.4). Then the 3'UTR (SEQ ID No.5) and 5'UTR (SEQ ID No.6) were amplified from human genomic DNA, and the polyA-containing sequence (SEQ ID No.7) was synthesized from the whole sequence, using overlapping PCR Methods All of them were connected to the DNA of the TERT CDS region to form a fragment: 5'UTR-TERT CDS-3'UTR-sequence containing polyA. Then, the fragment was double-digested with restriction endonucleases EcoR1 and SalI and ligated to the same digested plasmid Pcmv6-XL4. The telomerase plasmids with the correct sequence verified by sequencing, the target gene fragments in these plasmids are linearized by en...
Embodiment 2
[0054] Example 2 Detection of chondrocyte proliferation, telomerase activity, telomere length and cell metabolism after treatment with telomerase mRNA and complete medium
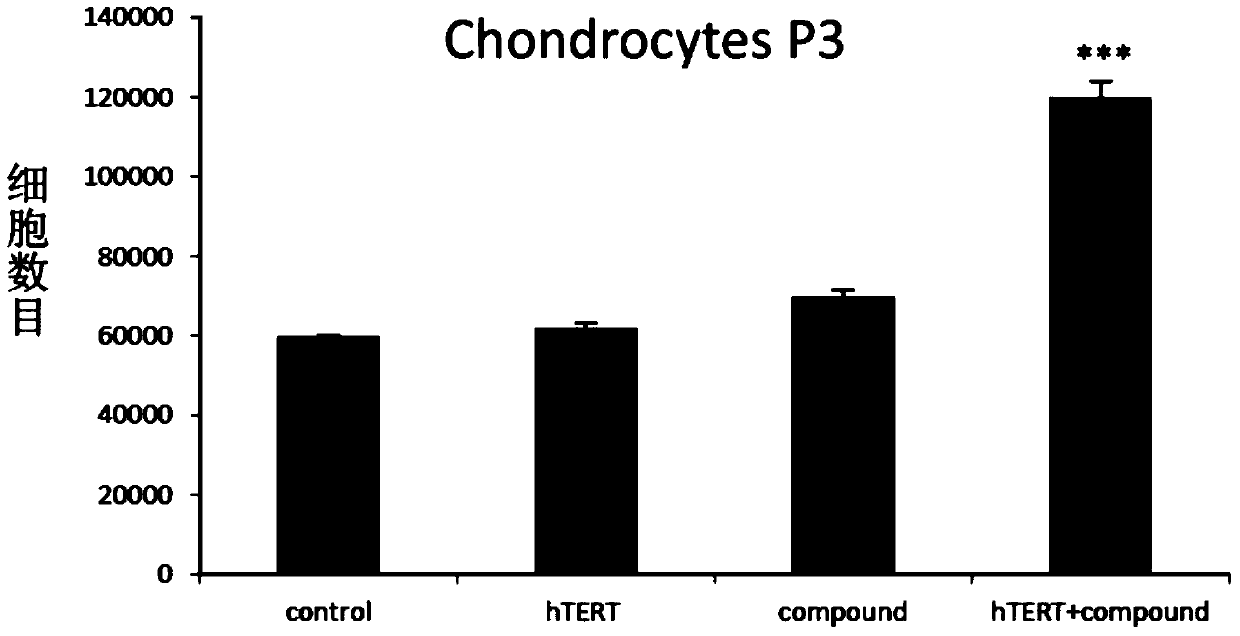
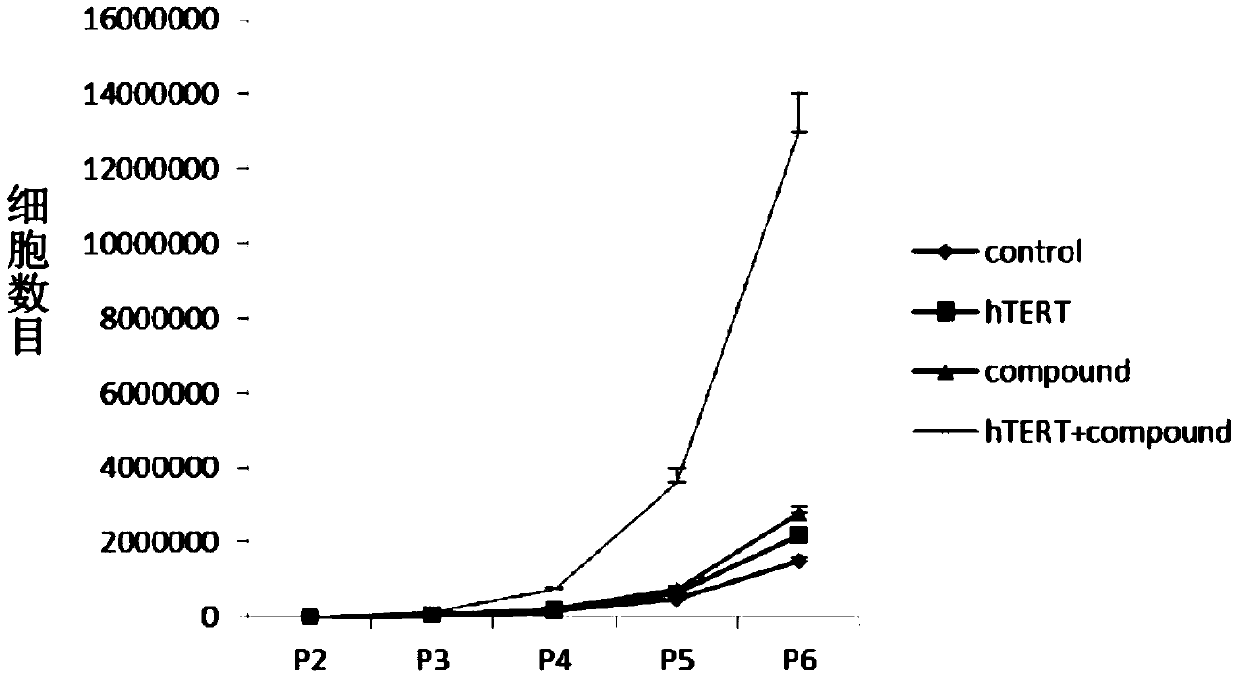
[0055] In this example, 4 groups of experiments are set up, namely control, hTERT, Compound and hTERT+compound, wherein control represents cells without any treatment, using basal medium (DMEM / F12 (Hyclone) + 5% human AB serum (Innovative)) Culture; hTERT represents cells transfected with telomerase mRNA, cultured in basal medium; Compound represents cells not transfected with telomerase mRNA, cultured in complete medium supplemented with various small molecule compounds; hTERT+compound represents cells Transfect telomerase mRNA and culture in complete medium. The primary chondrocytes were the zeroth generation, and at the third generation, the cells were planted in the wells of a 6-well plate, with 20,000 P3 generation chondrocytes per well.
[0056] When the cell density reaches nearly 100%, digest the c...
Embodiment 3
[0063] Example 3 Assembly of human tissue engineered regenerated cartilage
[0064] After the chondrocytes of elderly patients treated with telomerase mRNA and cultured in the complete medium containing various small molecular compounds are expanded to a sufficient number and in good growth state, it is necessary to prepare cell suspensions on collagen membrane scaffolds. After assembly and compounding, when the chondrocytes are completely attached to the collagen membrane, the finished human tissue engineered regenerated cartilage is made and transported to the hospital for treatment.
[0065] Firstly, AM medium (DMEM / F12 (Hyclone)+10% patient's autologous serum) was prepared. The cultured chondrocytes were taken for cell digestion, digested with 0.05% trypsin at 37°C for 5 min, and added complete medium to terminate the digestion. Centrifuge at 300g for 5 minutes at room temperature, resuspend the cells in AM medium, and count the cells. Centrifuge at 1500rpm for 5min, rem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com