System and method for preparing durene by utilization of toluene and methanol
A technology for the preparation of durene and methanol, which is applied in chemical instruments and methods, condensation between hydrocarbons and non-hydrocarbons, purification/separation of hydrocarbons, etc., which can solve the problem of limited raw material sources, high raw material costs, and low yields and other problems to achieve the effect of solving the low purity and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
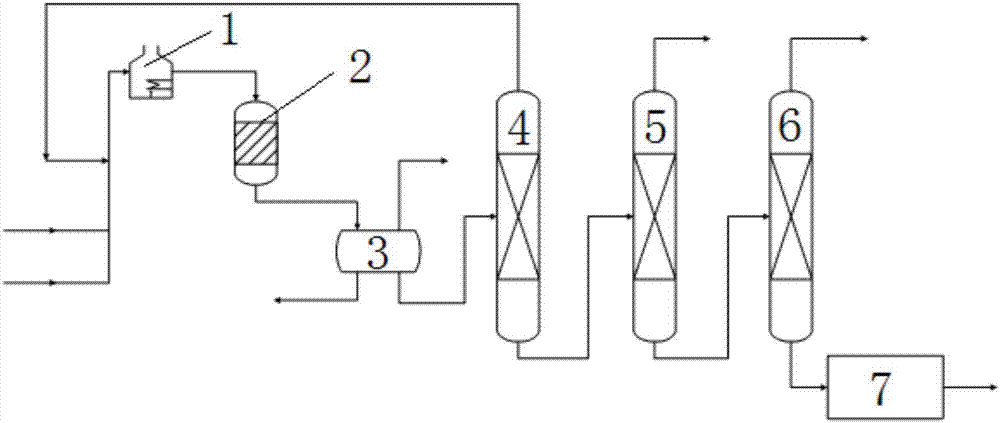
[0040] Toluene and methanol reach 350°C after being preheated by preheater 1, and then the preheated toluene and methanol are transported into alkylation reactor 2 for reaction. The mass flow rate of raw material gas toluene and methanol is 4234kg / h, and the reaction temperature is 390°C, reaction pressure 5.0MPa, mass space velocity 3h -1 , the reaction catalyst is HZSM-5 molecular sieve catalyst, and the reaction residence time is 3s; the reaction liquid is separated from oil and water to remove water and dry gas, and the separated waste liquid is sent to the waste water treatment station for treatment, and the separated dry gas is passed into the heating furnace for combustion, which is The heat is provided by the alkylation reactor, and the separated oil phase enters the first rectification tower 4, the temperature at the top of the tower is 100°C, rectification under normal pressure, the top components are recovered to the preheater 1, after heating Enter the alkylation r...
Embodiment 2
[0042] Toluene and methanol reach 330°C after being preheated by preheater 1, and then the preheated toluene and methanol are transported into alkylation reactor 2 for reaction. The mass flow rate of raw material gas toluene and methanol is 7465kg / h, and the reaction temperature is 360°C, reaction pressure 4.0MPa, mass space velocity 4h -1 , the reaction catalyst is HZSM-5 molecular sieve catalyst, and the reaction residence time is 4s; the reaction liquid is separated from oil and water to remove water and dry gas, and the separated waste liquid is sent to the waste water treatment station for treatment, and the separated dry gas is passed into the heating furnace for combustion. The heat is provided by the alkylation reactor, and the separated oil phase enters the first rectification tower 4, the temperature at the top of the tower is 100°C, rectification under normal pressure, the top components are recovered to the preheater 1, after heating Enter the alkylation reactor 2 ...
Embodiment 3
[0044] Toluene and methanol are preheated by preheater 1 to reach 340°C, and then the preheated toluene and methanol are transported into alkylation reactor 2 for reaction, the mass flow rate of raw material gas toluene and methanol is 3578kg / h, and the reaction temperature is 380 ℃, the reaction pressure is 3.0MPa, and the mass space velocity is 2h -1 , the reaction catalyst is HZSM-5 molecular sieve catalyst, and the reaction time is 5s; the reaction liquid is separated from oil and water to remove water and dry gas, and the separated waste liquid is sent to the waste water treatment station for treatment, and the separated dry gas is passed into the heating furnace for combustion to produce alkane The basement reactor provides heat, and the separated oil phase enters the first rectification tower 4, the temperature at the top of the tower is 100°C, rectification under normal pressure, and the top components are recovered to the preheater 1, and then enter the The reaction i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com