Protein bioluminescence imaging sensor based on perylene bisimide star-shaped polymers
A multi-arm polymer, imaging sensor technology, applied in the field of analytical chemistry, can solve the problems of the background matrix not having the possibility to develop into a portable device, unfavorable sensor deviceization, etc., to avoid biological matrix interference, low cost, and stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
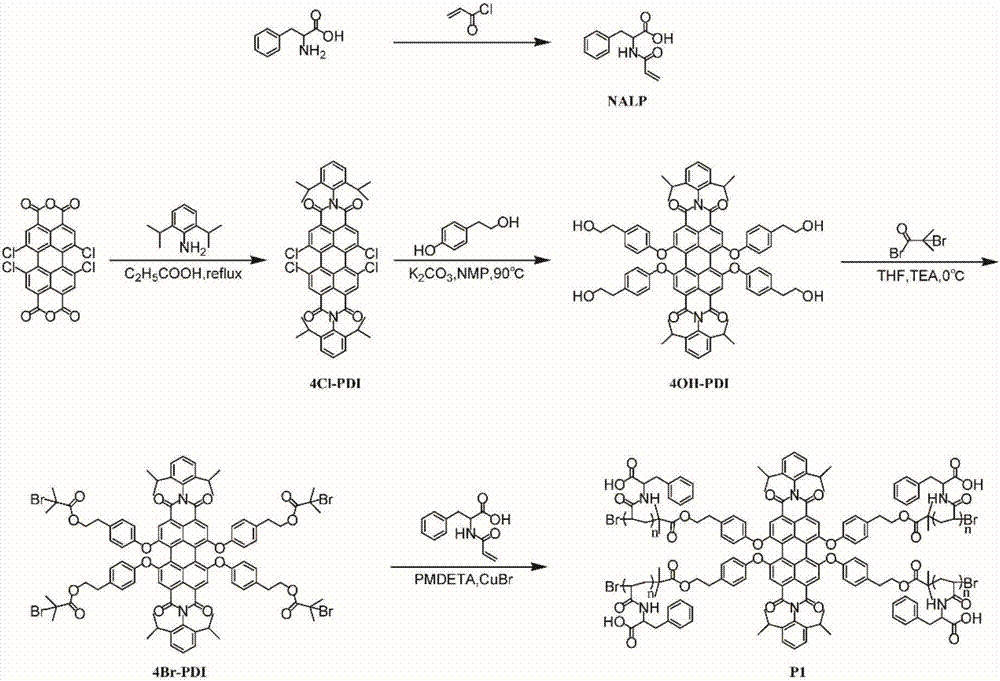
[0042] Example 1: Preparation of perylene imide polymer monomer.
[0043] (1) Preparation of N-acryloyl-L-phenylalanine (NALP): 3.3038g phenylalanine (0.02mol), 0.01g2,6-di-tert-butyl-p-cresol, 0.8g NaOH (0.02 mol) and 20mL of distilled water were placed in a 100ml round bottom flask to obtain a clear solution (pH12), stirred in an ice bath, and 1.63ml of acryloyl chloride (0.02mol) was added dropwise to the solution. After the dropwise addition was complete, the mixture was allowed to warm up to room temperature and stirred at room temperature for 1 hour. The clear aqueous solution was then acidified to pH 1-2 with concentrated hydrochloric acid. The precipitate was filtered and recrystallized, yield: 62%.
[0044] (2) N-acryloyl-L-serine (NALS), N-acryloyl-L-aspartic acid (NALA), N-acryloyl-L-leucine (NALL) and N-acryloyl-L - The preparation of histidine (NALH) all uses 0.02 mol of the corresponding amino acid as a raw material, following the same preparation steps as NAL...
Embodiment 2
[0046] Example 2: Preparation of peryleneimide multi-arm polymers P1-P6.
[0047] 6 kinds of perylene imide multi-arm polymers, i.e. P1, P2, P3, P4, P5 and P6, are prepared as follows:
[0048] Preparation of P1: The polymerization reaction was carried out in a strictly dry Schlenk tube, to which the initiator 4Br-PDI (10 mg, 5.4 × 10-3 mmol, 1 equivalent), NALP (508 mg, 2.16 mmol, 4 × 100 equivalents) and butyl Ketone / methanol / water (2:1:1, total 2.0 mL). The reaction tube was degassed by three freeze-pump-thaw cycles before addition of pentamethyldivinyltriamine (77.8 mg, 0.45 mmol, 4 x 21 equiv) and CuBr (21.4 mg, 0.15 mmol, 4 x 7 equiv). After stirring at room temperature for 10 minutes to ensure that the catalyst had completely formed a complex, polymerization was carried out at 60° C. under nitrogen protection. After reacting for 5 hours, the reaction was quenched with liquid nitrogen, the reaction mixture was poured into excess diethyl ether to precipitate, and the pr...
Embodiment 3
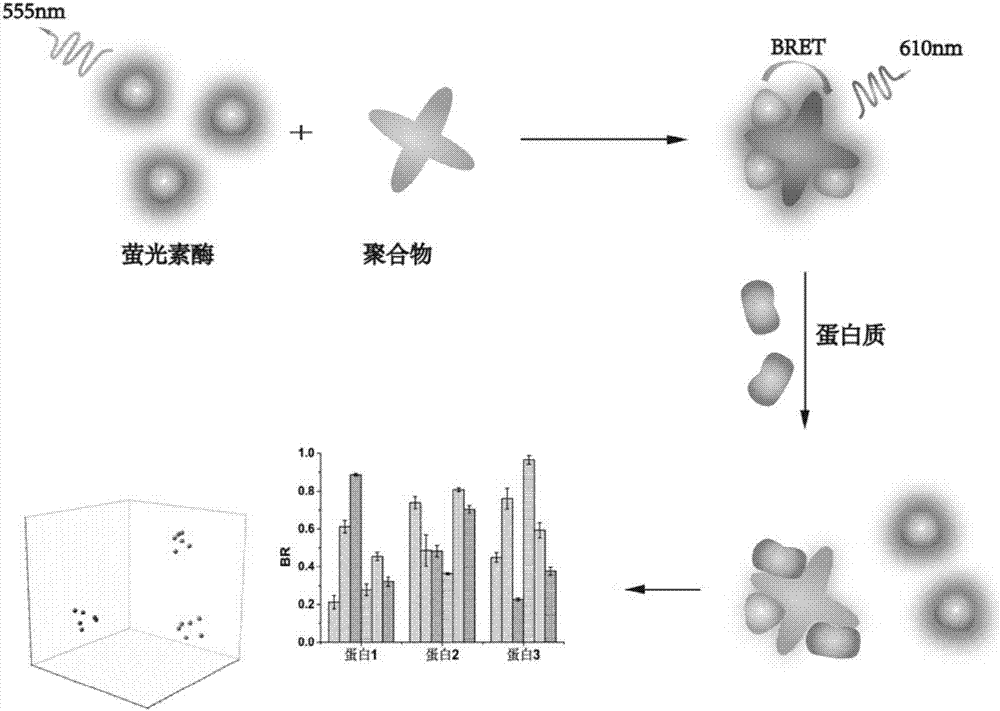
[0060] Example 3: Protein bioluminescent imaging sensor based on peryleneimide multi-arm polymer
[0061] Please refer to the appendix for the overlap of the luciferase emission spectrum and the excitation spectrum of the polymer P1-P6 Figure 9 A, the results of detecting BRET signal in the detection wavelength range of 500-700nm are as follows Figure 9 Shown in B.
[0062] Construct a sensing unit, the sensing unit is a BRET acceptor peryleneimide multi-arm polymer and a BRET donor luciferase, and a titration method is used to determine the ratio of the BRET acceptor perylene imide multi-arm polymer to the donor luciferase. The stoichiometric ratio of luciferase makes the luciferase concentration constant, gradually increases the polymer concentration, and records the change of BRET efficiency. When the curve becomes stable and no longer rises, the BRET efficiency at this point is the maximum BRET efficiency. The stoichiometric ratio of BRET acceptor and BRET donor lucife...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com