Ibuprofen cubic liquid crystal precursor solution, cubic liquid crystal nanoparticles and preparation method of cubic liquid crystal nanoparticles
A technology of liquid crystal precursors and nanoparticles, applied in the field of cubic liquid crystal nanoparticles and its preparation, ibuprofen cubic liquid crystal precursor solution, can solve the problems of low solubility, short half-life, etc., achieve strong hydrophobicity, poor water solubility, overcome Effects of Disorders of Dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation method of the ibuprofen cubic liquid crystal nanoparticles of the present embodiment comprises the following steps:
[0053] Weigh ibuprofen, F127 and phytantriol according to the raw material prescription shown in Table 1, place them in a 100mL plastic centrifuge tube, heat and melt in a water bath at 60°C, vortex, mix well, and place at room temperature for 2 days to obtain Ibuprofen Cubic Liquid Crystal Precursor Solution.
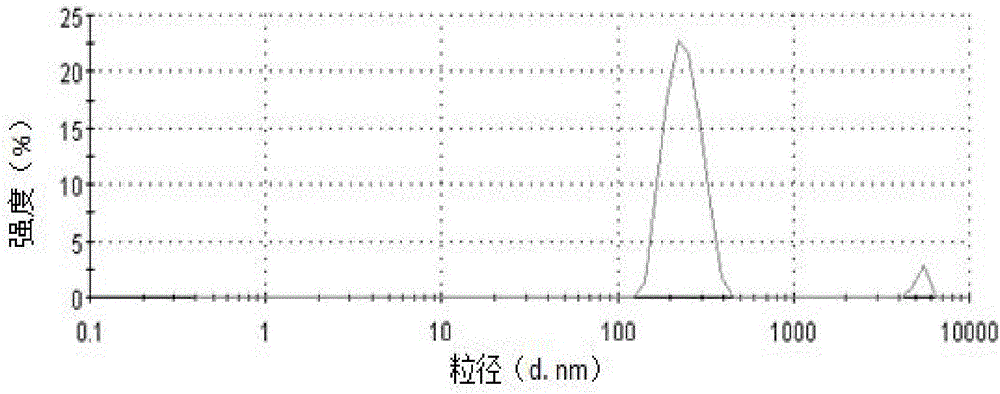
[0054] Add 30mL of water to the ibuprofen cubic liquid crystal precursor solution, carry out ultrasonic dispersion with an ultrasonic pulverizer, the time is 10min, the ultrasonic power used is 200W, the ultrasonic working time is 5s, and the intermittent time is 10s to obtain the ibuprofen cubic liquid crystal coarse dispersion solution , the particle size distribution in the coarsely dispersed solution is uneven, and there are micron-sized particles (see figure 1 ).
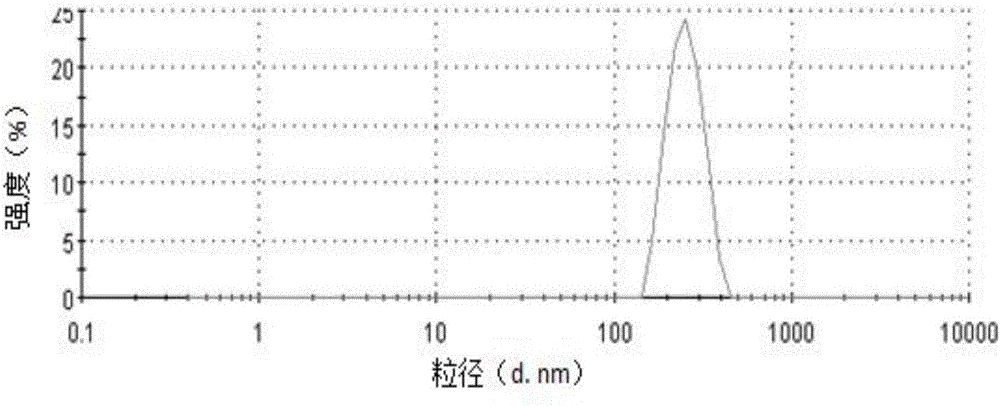
[0055] The ibuprofen cubic liquid crystal coarse dispersion...
Embodiment 2
[0065] With the raw material prescription shown in Table 2, according to the preparation method of Example 1, prepare ibuprofen cubic liquid crystal nanoparticle solutions with different stabilizer types and dosages.
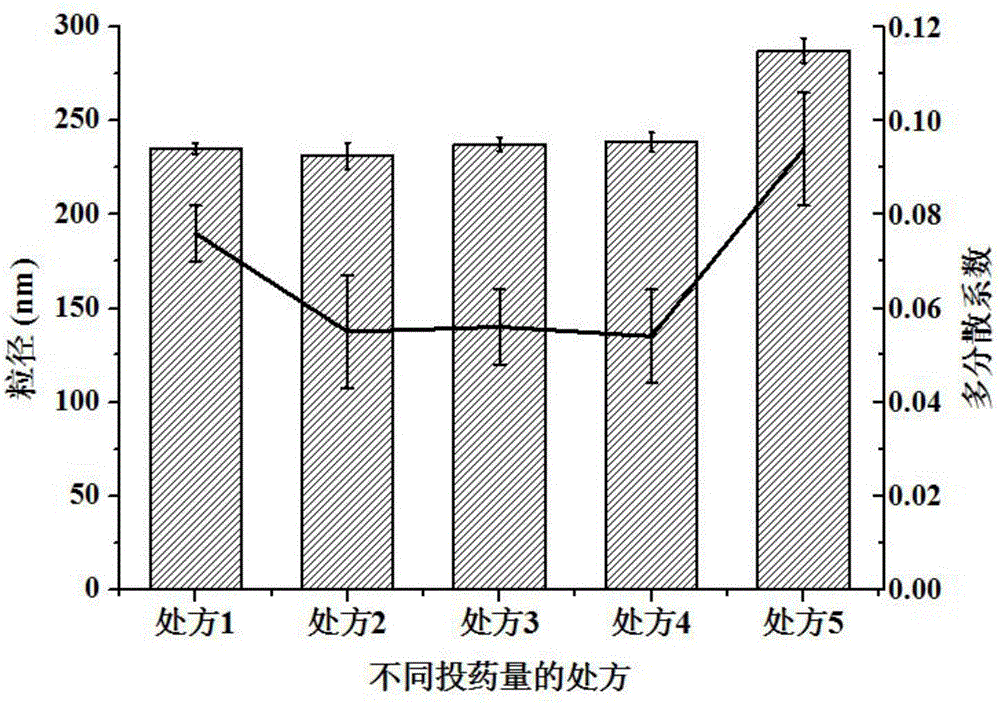
[0066] Measure the particle diameter of the ibuprofen cubic liquid crystal nanoparticles prepared in this embodiment, and the measurement method is the same as in Example 1, and judge its stability by comparing the particle diameter and PDI of the ibuprofen cubic liquid crystal nanoparticles on the 1st day and the 3rd day . see results Figure 5 , Table 2 and Table 3. As can be seen from the results, in the absence of a stabilizer, the nanoparticles are prone to aggregation and the particle size increases; the effect of the ibuprofen cubic liquid crystal coarse dispersion solution prepared by different amounts of F68 (poloxamer 188) is not good enough, and it is easy to agglomerate and the ibuprofen cubic liquid crystal coarse dispersion solution prepared by d...
Embodiment 3
[0073] Take ibuprofen, phytantriol and F127 by mass ratio 12:100:10, prepare ibuprofen cubic liquid crystal nanoparticles according to the method of Example 1, the difference is that the homogeneous pressure adopted is 670bar, 800bar, 1200bar and 1500bar; Homogenization times are 3, 5 and 9 times respectively.
[0074] Measure the ibuprofen cubic liquid crystal nanoparticles prepared in this embodiment, and the determination method is the same as that in Example 1. The results are shown in Table 4. The result shows, under the condition of different homogenization pressure and number of times, ibuprofen cubic liquid crystal nano-particle all has smaller particle diameter and PDI, wherein, when homogenization pressure is 1200bar, when homogenization number of times is 9 times, particle diameter minimum. Therefore, the optimal conditions for the high-pressure homogenization of ibuprofen cubic liquid crystal nanoparticles are: homogenization pressure 1200 bar, homogenization cyc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com