Antitumor compound targeting FAP-alpha enzyme and preparation method and application thereof
A compound and anti-tumor technology, applied in the direction of anti-tumor drugs, organic chemical methods, active ingredients of heterocyclic compounds, etc., can solve the problems of limited application, achieve the effects of reducing toxicity and in vivo toxicity, high product purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of embodiment of the preparation method of Z-GP-procarbazine of the present invention, described preparation method comprises the following steps:
[0046] 1.1.1 Preparation of N-isopropyl p-toluamide
[0047] Weigh 273mg (2.0mmol) of p-toluic acid into a 25mL round bottom flask, add 5.0mL (68.8mmol) of thionyl chloride (excess), heat and reflux in an oil bath at 78°C for 2h; remove excess dichloride by rotary evaporation Sulfone to obtain p-toluamide; re-dissolve p-toluamide in 2.0 mL of dry dichloromethane (DCM); another 1.0 mL (11.7 mmol) of isopropylamine was dissolved in 2.0 mL of dry DCM , to obtain liquid A; control the temperature at 30°C, gradually add liquid A dropwise into the reaction system, continue stirring for 30 minutes after the addition, and stir for 3 hours at room temperature; then raise the temperature to 40°C, continue the reaction for 30 minutes, stop heating; The solution was poured into 50 mL of ice water, extracted 4 times with dichlor...
Embodiment 2
[0061] Example 2 Investigation experiment of Z-GP-Pcb targeted release characteristics
[0062]Experimental method: HPLC chromatographic conditions are as follows: high performance liquid chromatograph Agilent 1200; Chromatographic column CosmosilC18 reverse-phase chromatographic column (4.6 * 250mm, 5 μ m); Mobile phase (0min, 55% methanol and 45% water (containing 2mM ammonium formate); 10min, 65% methanol and 35% water (containing 2mM ammonium formate); 15min, 75% methanol and 25% water (containing 2mM ammonium formate); 30min, 85% methanol and 15% water (containing 2mM ammonium formate); 40min, 85% methanol and 15% water (containing 2mM ammonium formate); flow rate 1mL / min; detection wavelength: 254nm; injection volume 2μL.
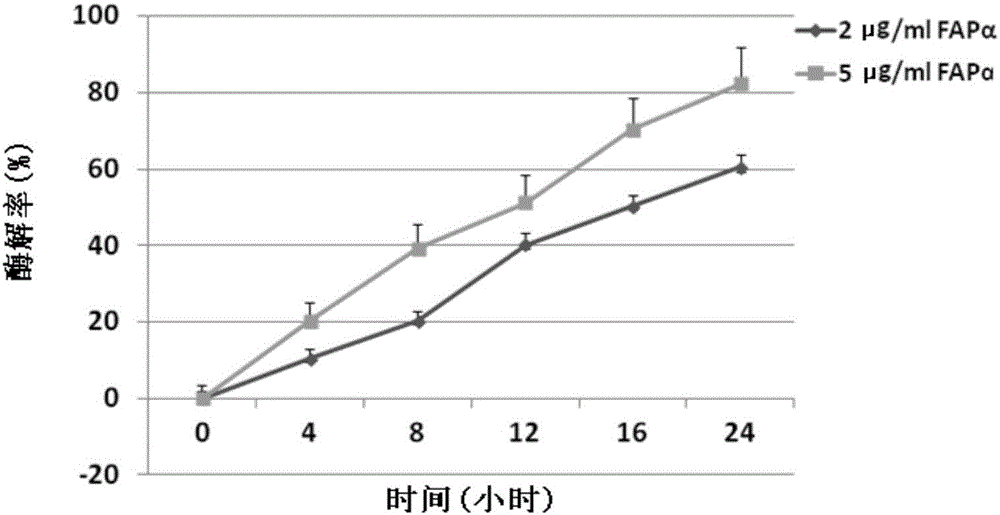
[0063] The method for establishing the Z-GP-procarbazine detection standard curve is as follows: dissolve Z-GP-procarbazine in the enzymolysis buffer (50mM Tris-HCl, 1.0M NaCl, pH 7.4), and set 5 concentration gradients of 0.012725 . 2μg / ml and 5μg / ...
Embodiment 3
[0065] Example 3 Investigation experiment on the change of cytotoxicity before and after enzymatic hydrolysis of Z-GP-Pcb
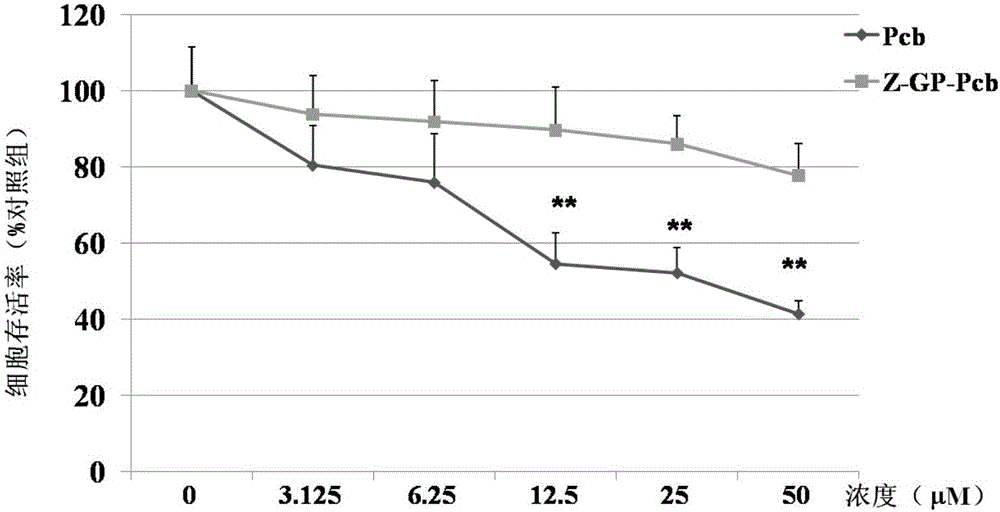
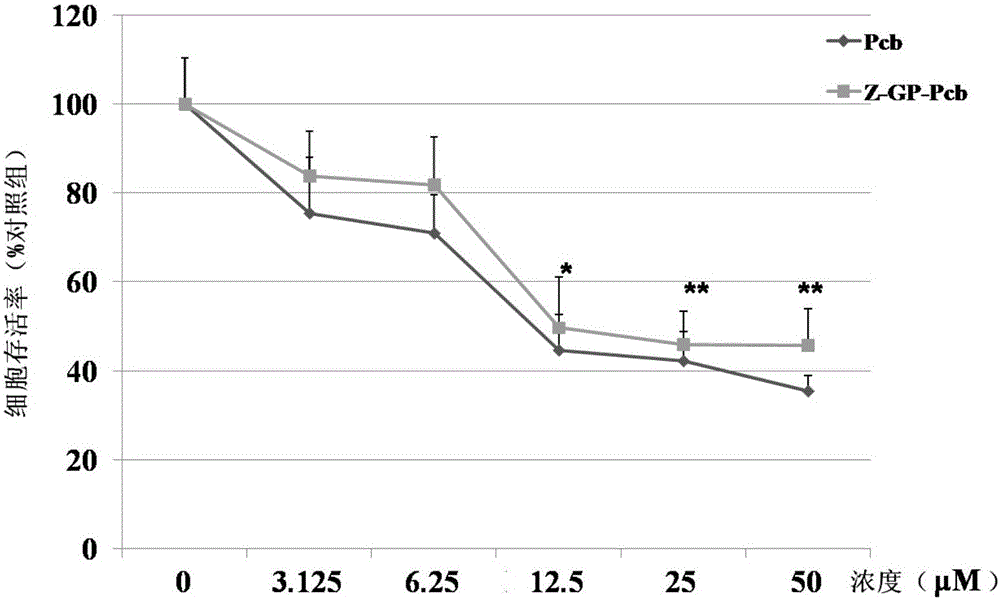
[0066] Experimental method: Z-GP-Pcb and Pcb were treated with NCI-H460 cell line (purchased from Shanghai Cell Bank, Chinese Academy of Sciences) for 48 hours in the drug concentration range of 0.1-50 μM, and the cytotoxicity of the two was compared by MTT method; Z-GP-Pcb was co-incubated with FAP-alpha, and the supernatant of the incubation was taken to treat the NCI-H460 cell line for 48 hours. The effect of FAP-alpha enzymolysis on the cytotoxicity of Z-GP-Pcb was analyzed by MTT method. Whether Z-GP-Pcb can restore its potential cytotoxicity under enzymatic hydrolysis.
[0067] Experimental results: The results of cytotoxicity experiments showed that the cytotoxicity of Z-GP-Pcb to NCI-H460 was significantly lower than that of Pcb ( figure 2 ); under the action of FAP-alpha enzymolysis, Z-GP-Pcb restores its cytotoxicity ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com