Drug delivery system for targeting co-delivery of photosensitizer and chemotherapeutic drug
A chemotherapeutic drug and photosensitizer technology, applied in the field of new drug delivery system, can solve the problems of reducing drug loading and encapsulation efficiency, high drug accumulation, poor stability of photosensitizer, etc., to achieve high drug loading efficiency in the inner core, low Critical micelle concentration, effect of increasing accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation and characterization of nanoparticles co-encapsulating doxorubicin and chlorin e6
[0032] Preparation of nanoparticles by nanoprecipitation method, including: weighing doxorubicin hydrochloride and adding it to the mixture of acetone and tetrahydrofuran, and adding triethylamine (the molar ratio of doxorubicin to triethylamine is 1:3) to seal the reaction overnight, and the reaction The liquid was spin-dried in a vacuum to obtain desalted doxorubicin, which was co-dissolved in acetone (1 mL) with TPGS-Ce6, TPGS-PLA, and TPGS at a mass ratio of 6:3:1 to prepare a 10 mg / mL polymer acetone solution. Add the polymer solution dropwise into rapidly stirred 0.1M phosphate buffer (pH 7.4) with a 1mL syringe at room temperature (volume ratio of organic phase to aqueous phase is 1:2), and after rapid stirring for 3 h, use coagulation The gel column was used to separate and remove unencapsulated doxorubicin to obtain NP, which was stored at 4°C for later use...
Embodiment 2
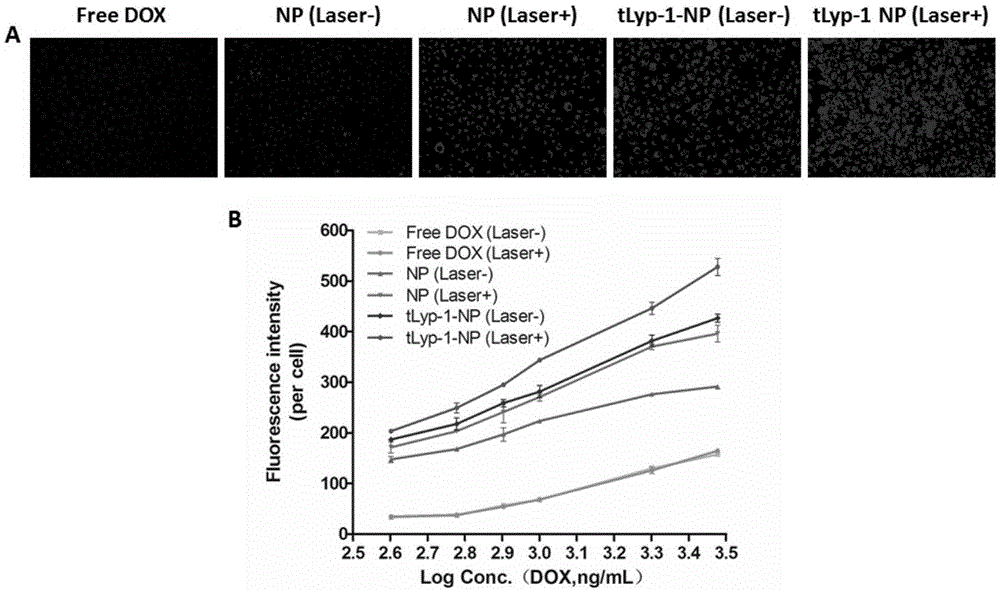
[0034] Qualitative and quantitative results of nanoparticle uptake by embodiment 2 HUVEC cells
[0035] HUVEC cells were seeded in a 96-well plate at a density of 5000 cells / well, placed in a 5% carbon dioxide incubator and cultured for 24 hours, and the original medium was replaced with doxorubicin group, NP group, NP+light group, tLyP-1- NP group, tLyP-1-NP+ light group, and set the concentration gradient to 0.4, 0.6, 0.8, 1, 2, 3 μg / mL, after 30 minutes of dark incubation, use a laser probe emitting 660nm near-infrared wavelength at 0.6 J / cm 2 (22mW / cm 2 , 30s) to irradiate the well plate, continue to incubate in the incubator at 37°C for 2h, wash the plate 3 times with warm PBS (0.1M phosphate buffered saline solution, pH7.4), add paraformaldehyde Fix for 15 minutes, stain the nucleus with 2 μg / mL Hochest33258 for 10 minutes, and finally add 200 μL PBS to each well to quantitatively analyze the cellular uptake of the drug with a high-content instrument; observe and take pi...
Embodiment 3
[0037] Qualitative and quantitative results of nanoparticle uptake by embodiment 3MCF-7 / ADR cells
[0038] MCF-7 / ADR cells were seeded in 96-well plates at a density of 5000 / well, placed in a 5% carbon dioxide incubator and cultured for 24 hours, and the original medium was replaced with doxorubicin group, NP group, NP+light group, tLyP-1-NP group, tLyP-1-NP+light group, and set the concentration gradient to 0.4, 0.6, 0.8, 1, 2, 3 μg / mL, after 30min of dark incubation, use a laser emitting 660nm near-infrared wavelength Probe at 0.6J / cm 2 (22mW / cm 2 , 30s) to irradiate the well plate, continue to incubate at 37°C for 2 hours, wash the plate three times with warm PBS, add paraformaldehyde to fix for 15 minutes, 2 μg / mL Hochest33258 to stain the nuclei for 10 minutes, and finally Add 200 μL PBS to each well, and use a high-content instrument to quantitatively analyze the cellular uptake of the drug; observe and take pictures with an inverted fluorescence microscope;
[0039] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com