A kind of modified bismaleimide resin and preparation method thereof
The technology of bismaleimide resin and bismaleimide is applied in the directions of ether preparation, ester reaction preparation of ether, chemical instruments and methods, etc., and can solve the problems of large loss of mechanical properties, poor bending properties and the like, Achieve the effect of fewer preparation steps, fewer conversion steps, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of 5,5'-methylene bis(1-allyl ether-2-methoxy-4-methylbenzene)
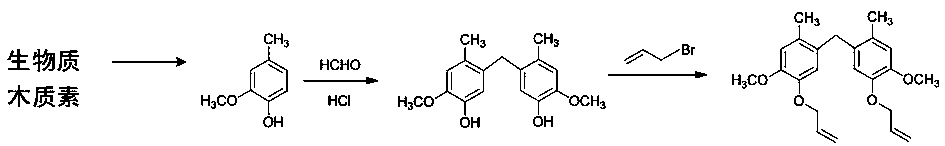
[0029] See attached figure 1 , which is a reaction schematic diagram of synthesizing 5,5'-methylene bis(1-allyl ether-2-methoxy-4-methylbenzene) according to the technical scheme of the present invention. Attached figure 1 The process steps provided, the specific method is as follows:
[0030] 100g lignin and 1g CuSO 4 (Catalyst) Add 1000g of sodium hydroxide solution (pH 12), reflux at 90°C for 3 hours; after the reaction, cool the system to 70°C naturally, add 1000 parts of sulfuric acid solution with pH 1 to the reaction system , Reflux reaction 0.5h. The resulting solution was extracted with ethyl acetate, and the ethyl acetate was rotary evaporated to obtain crude vanillin. And then purified by water crystallization to obtain white needle crystals, which is pure vanillin. In the technical solution of the present invention, the preparation of vanillin can refer to literature: Li...
Embodiment 2
[0051] (1) Preparation of 5,5'-methylene bis(1-allyl ether-2-methoxy-4-methylbenzene)
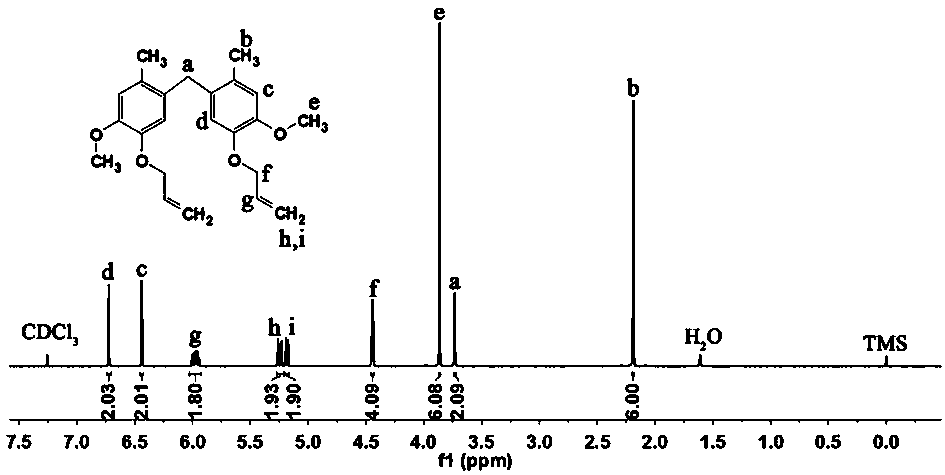
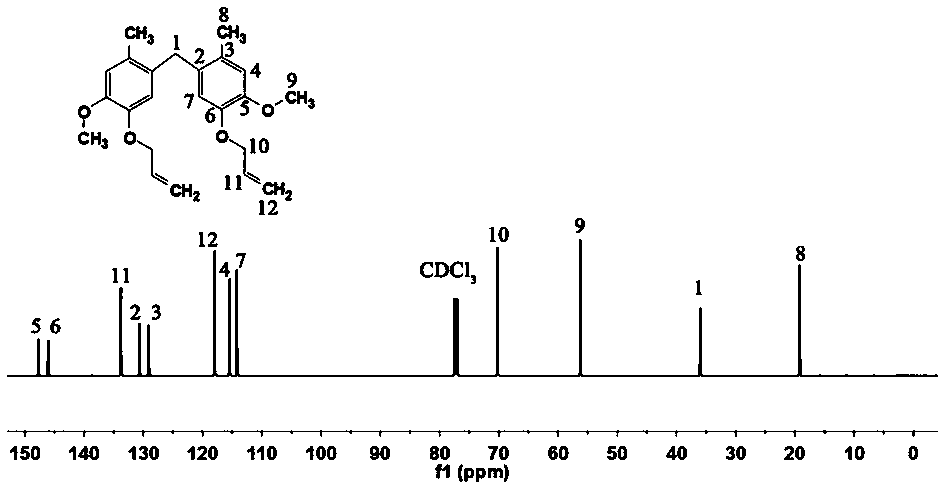
[0052] Under nitrogen protection, at 20°C, 138.16g of 2-methoxy-4-methylphenol and 48.7g of 35% formaldehyde solution were mixed, diluted with deionized water, and then 250mL of concentrated hydrochloric acid was slowly added. The reaction was refluxed at 110°C for 3 hours, and the obtained crude product was washed with 5% ethanol solution and dried to obtain 5,5'-methylenebis(2-methoxy-4-methylphenol).
[0053] Dissolve 144.17g of 5,5'-methylenebis(2-methoxy-4-methylphenol), 2.04g of sodium dodecylsulfonate and 127.03g of bromopropene in 580mL of absolute ethanol, and stir; The above solution was heated up to 45°C, and 533.34g of 20% NaOH aqueous solution was slowly added dropwise. After the dropwise addition, the temperature was raised to 75°C and kept stirring for 4 hours. Cool down after the reaction, extract the solution with ethyl acetate, and remove the solvent by rotary evaporation...
Embodiment 3
[0057] (1) Preparation of 5,5'-methylene bis(1-allyl ether-2-methoxy-4-methylbenzene)
[0058] Under nitrogen protection, at 20°C, 138.16g of 2-methoxy-4-methylphenol and 40.58g of 37% formaldehyde solution were mixed, diluted with deionized water, and then 275mL of concentrated hydrochloric acid was slowly added. The reaction was refluxed at 120°C for 4 hours, and the obtained crude product was washed with 10% ethanol solution and dried to obtain 5,5'-methylenebis(2-methoxy-4-methylphenol).
[0059]Dissolve 144.17g of 5,5'-methylenebis(2-methoxy-4-methylphenol), 2.04g of sodium dodecylsulfonate and 127.03g of bromopropene in 580mL of absolute ethanol, and stir; The above solution was heated up to 50°C, and 533.34g of 15% NaOH aqueous solution was slowly added dropwise. After the dropwise addition, the temperature was raised to 75°C and kept stirring for 4 hours. Cool down after the reaction, extract the solution with ethyl acetate, and remove the solvent by rotary evaporatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bending strength | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com