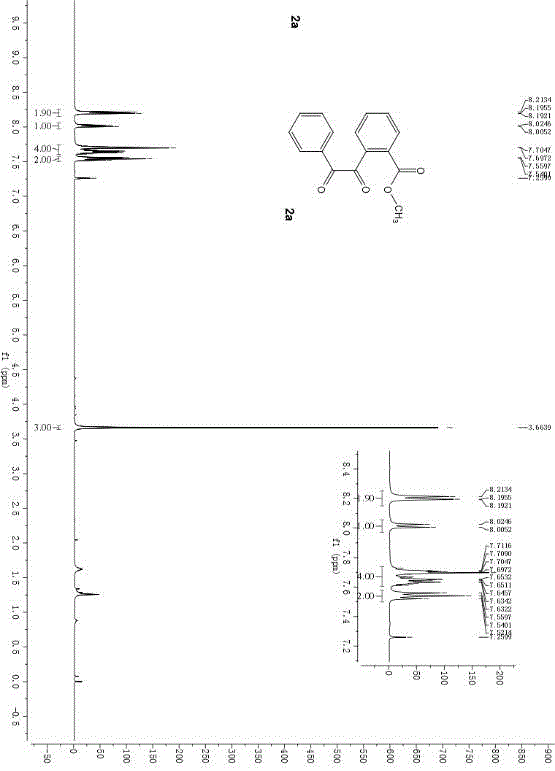
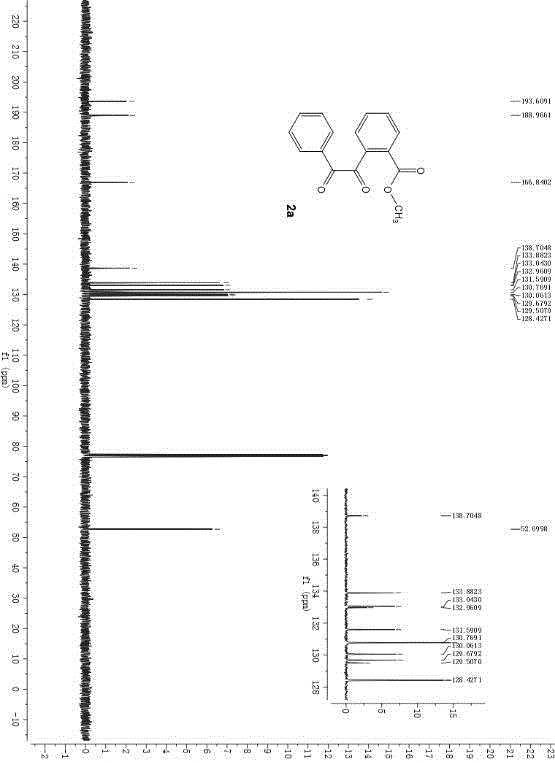
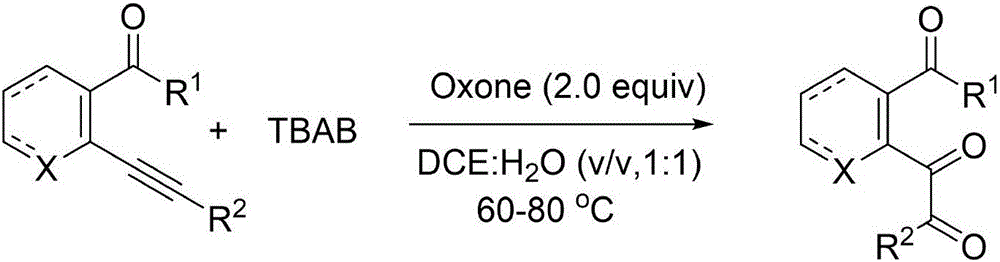
Method for synthesizing ph-dicarbonyl-aryl-formic-ether series compounds based on metal-free participation method
A technology of dicarbonyl aryl formate and dicarbonyl aryl formate, which is applied in the field of metal-free preparation of o-dicarbonyl aryl formate series compounds, can solve the problem of low atom utilization and achieve substrate Wide applicability, less by-products, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] In the air atmosphere, adopting 1.0 parts by weight of o-phenylethynyl benzoate as a reaction substrate, adding 2.0 parts by weight of tetra-n-butylammonium bromide as a bromine source and 2.0 parts by weight of potassium peroxymonosulfonate as an oxidant , adding an aqueous solution of 1,2-dichloroethane with a volume fraction of 50%, so that the concentration of o-phenylethynyl benzoate is 0.1M. After reacting for 6 hours at a temperature of 60° C., 10 parts by weight of silica gel was added and the mixture was stirred for 0.5 hours. During the reaction, TLC was used to monitor until the reaction was complete. After the reaction, the reaction solution was filtered, the filtrate was extracted with ether, and the organic phase was extracted with anhydrous Na 2 SO 4 After drying and filtering again, the filtrate was distilled to remove the organic solvent and then flash column chromatography to obtain the pure product o-dicarbonyl aryl formate series compoun...
Embodiment 2
[0046]
[0047] In the air atmosphere, 1.0 parts by weight of ethyl 2-phenylethynyl-3-pyridinecarboxylate is used as a reaction substrate, and 2.0 parts by weight of potassium bromide is added as a bromine source and 2.0 parts by weight of potassium peroxymonosulfonate as an oxidant , adding an aqueous solution of 1,2-dichloroethane with a volume fraction of 50%, and the concentration of ethyl 2-phenylethynyl-3-pyridinecarboxylate was 0.1M. After reacting for 8 hours at a temperature of 70° C., 10 parts by weight of silica gel was added and the mixture was stirred for 0.6 hours. During the reaction, TLC was used to monitor until the reaction was complete. After the reaction, the reaction solution was filtered, the filtrate was extracted with ether, and the organic phase was extracted with anhydrous Na 2 SO 4 After drying and filtering again, the filtrate was distilled to remove the organic solvent and then flash column chromatography to obtain the pure product o-dicarbonyl...
Embodiment 3
[0053]
[0054] In the air atmosphere, adopt 1.0 parts by weight of 2-phenylethynyl-cyclohexenecarboxylate reaction substrate, add 2.5 parts by weight of tetra-n-butylammonium bromide as bromine source and 2.5 parts by weight of peroxymonosulfonic acid Potassium is an oxidizing agent, 1,2-dichloroethane aqueous solution with a volume fraction of 50% is added, and the concentration of ethyl 2-phenylethynyl-cyclohexenecarboxylate is 0.1M. After reacting for 9 hours at a temperature of 80° C., 10 parts by weight of silica gel was added and the mixture was stirred for 1 hour. During the reaction, TLC was used to monitor until the reaction was complete. After the reaction, the reaction solution was filtered, the filtrate was extracted with ether, and the organic phase was extracted with anhydrous Na 2 SO 4 After drying and filtering again, the filtrate was distilled to remove the organic solvent and then flash column chromatography to obtain the pure product o-dicarbonyl-aryl f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com