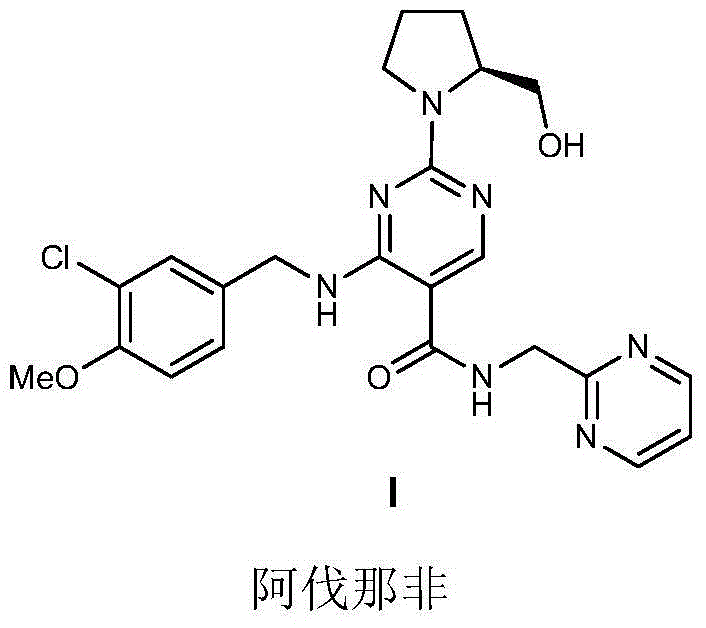
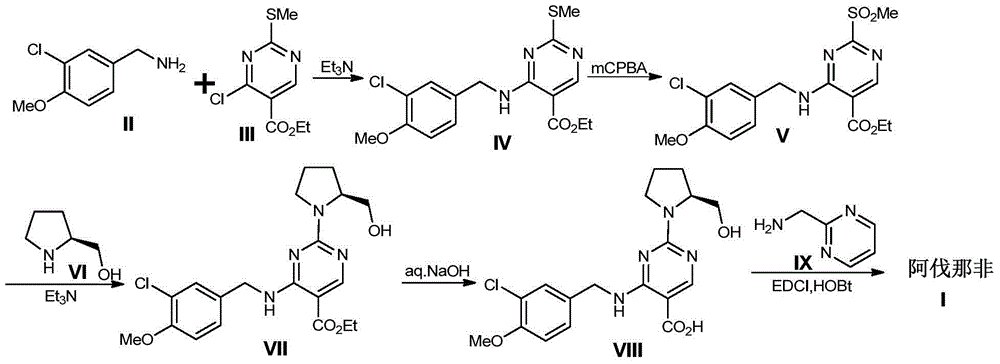
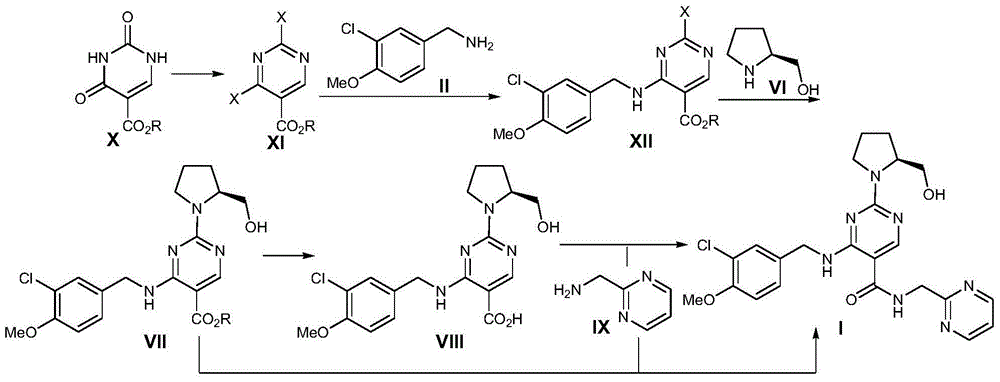
Preparation method of avanafil
A technology of avanafil and compounds, applied in the field of medicinal chemistry, can solve the problems of low reaction yield, many impurities, difficult to remove impurities, etc., and achieve the effect of simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of ethyl 2,4-dichloro-5-pyrimidinecarboxylate
[0059]
[0060] Into a 100ml three-neck flask, add ethyl uracil-5-carboxylate (2.2g, 11.95mmol) and N,N-dimethylaniline (2g, 16.50mmol). Phosphorus oxychloride (9.2 g, 60 mmol) was gradually added under ice-cooling, and stirred continuously. After the dropwise addition was completed, stir for 10 minutes, gradually raise the temperature to 110° C., and keep the temperature for 3 hours to react. The reaction solution was slowly poured into an ice-water mixture (100ml) to quench, and kept stirring to keep the temperature below 5°C. Extracted twice with ethyl acetate (200ml). The organic phases were combined, washed with saturated brine, dried and concentrated to obtain a crude product. The crude product was subjected to column chromatography to obtain 1.8 g of solid, with a yield of 68%.
[0061] 1 H NMR (DMSO-d 6 ,400MHz): δ9.13(s,1H), 4.37(q,2H), 1.32(t,3H).
Embodiment 2
[0062] Example 2 Preparation of tert-butyl 2,4-dichloro-5-pyrimidinecarboxylate
[0063]
[0064] Into a 100ml three-necked flask, add uracil-5-carboxylate tert-butyl ester (2.5g, 11.95mmol), N,N-dimethylaniline (2g, 16.50mmol). Phosphorus oxychloride (9.2 g, 60 mmol) was gradually added under ice-cooling, and stirred continuously. After the dropwise addition was completed, stir for 10 minutes, gradually raise the temperature to 110° C., and keep the temperature for 3 hours to react. Concentrate to dryness, add saturated sodium bicarbonate solution (100ml), keep the temperature below 5°C. Extracted twice with ethyl acetate (200ml). The organic phases were combined, washed with saturated brine, dried and concentrated to obtain a crude product. The crude product was subjected to column chromatography to obtain 1.9 g of solid, with a yield of 65%.
[0065] 1 H NMR (DMSO-d 6 ,400MHz): δ9.13(s,1H),1.43(s,9H). MS (m / z): 250 (MH + ).
Embodiment 3
[0066] Example 3 Preparation of ethyl 4-(3-chloro-4-methoxybenzylamino)-2-[(S)-2-hydroxymethylpyrrolidinyl-1-]-5-pyrimidinecarboxylate
[0067]
[0068] To a 25ml three-neck flask, add ethyl 2,4-dichloro-5-pyrimidinecarboxylate (150mg, 0.679mmol), triethylamine (270mg, 2.668mmol), dichloromethane (2ml) in sequence, and gradually stir until dissolved , cooled in an ice bath to below 0°C, 3-chloro-4-methoxybenzylamine hydrochloride (145 mg, 0.697 mmol) was added in batches to the reaction solution, and the reaction was kept for two hours after the addition was complete. Then, L-prolinol (105 mg, 1.038 mmol) was added dropwise to the reaction solution, and after the addition was completed, the reaction was continued to stir for 2 hours. The reaction liquid was added to water (30 ml), and extracted with dichloromethane. The organic phases were combined, washed twice with water, dried over anhydrous sodium sulfate, the desiccant was filtered off, and the organic phase was conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com