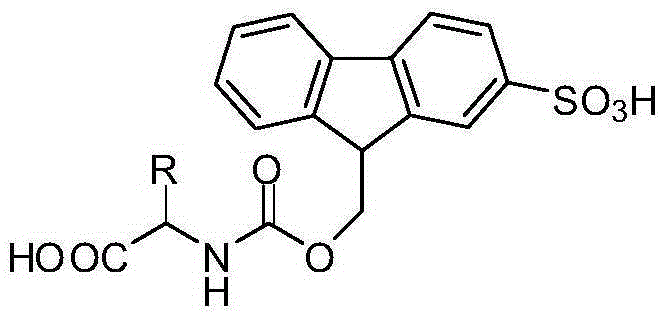
Fmoc (SO3H) protected amino acid having excellent hydrophilicity and preparation method thereof
A technology of SO3H and amino acids, which is applied in the field of preparation and purification of Fmoc-protected amino acids with good hydrophilicity and its preparation, and can solve the problems of poor hydrophilicity of polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
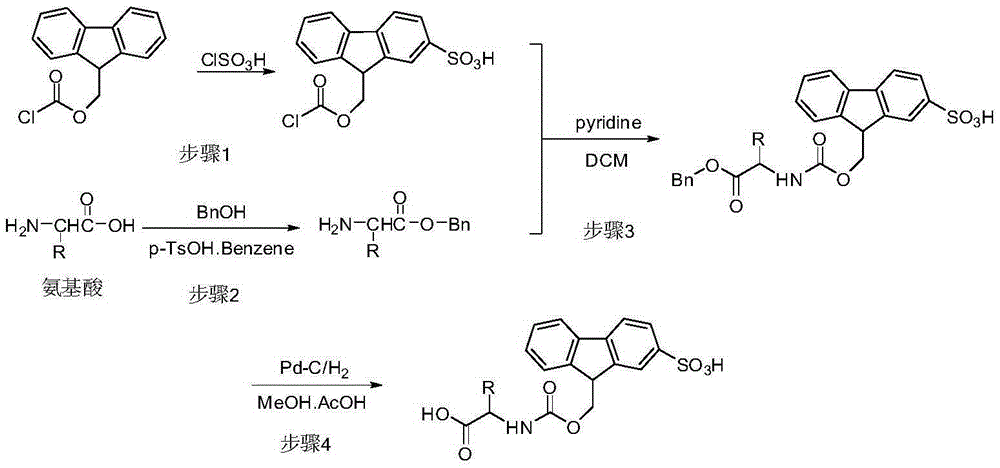
[0032] Example 1: Preparation of 9-((chlorocarbonyloxy)methyl)-9H-fluorene-2-sulfonic acid
[0033] Fluorenylmethoxycarbonyl chloride (64.75g) was dissolved in dichloromethane (500ml), and cooled to 0°C. A dichloromethane solution of methanesulfonyl chloride was slowly added dropwise (14 mL of methanesulfonyl chloride was dissolved in 250 mL of dichloromethane), and the temperature was controlled not to exceed 10° C. After the dropwise addition, stirring was continued for 3 hours. Cyclohexane (500 mL) was added to the reaction liquid. The reaction solution was centrifuged, and the obtained solid was washed three times with a mixed solvent (dichloromethane:cyclohexane=1:1, 750 mL×3) to obtain an off-white solid.
[0034] Structural data:
[0035] h 1 NMR: (D 3 COD)64.2-4.5(m,3,CHCH 2 ),5.4(s,H 2 O / RS0 3 H),7.1-7.9(m,6,aryl),8.1(s,1,H-1aryl)
Embodiment 2
[0036] Embodiment 2: the preparation of L-alanine benzyl ester
[0037] Dissolve L-alanine (3.5g) and p-toluenesulfonic acid (8.2g) in benzyl alcohol (16ml), add benzene (8ml) to the reaction solution, raise the temperature of the system to 100°C, reflux the benzene with water for about 15h. After the reaction solution was cooled to room temperature, diethyl ether (15 ml) was added, and then placed in the refrigerator overnight. Filtration afforded a white solid. The white solid was crystallized from acetone. The obtained crystals were dissolved in dichloromethane, washed three times with 5% aqueous sodium bicarbonate solution (20 mL×3), dried the organic phase with anhydrous sodium sulfate, filtered, and spun to remove the solvent to obtain a colorless oil.
Embodiment 3
[0038] Example 3: N-(9-(2-sulfonic acid)-fluorenylmethoxycarbonyl)-L-alanine benzyl ester
[0039]The product 9-((chlorocarbonyloxy)methyl)-9H-fluorene-2-sulfonic acid (7.6g) obtained in Example 1 was dissolved in a mixed solvent of ethyl acetate (10mL) and dichloromethane (150mL) . In addition, L-alanine benzyl ester (4 g) was weighed and dissolved in a mixed solvent of dichloromethane (100 mL) and pyridine (50 mL), and cooled to 0° C. in an ice bath. Slowly add the 9-((chlorocarbonyloxy)methyl)-9H-fluorene-2-sulfonic acid solution dropwise into the L-alanine benzyl ester solution, keeping the temperature below 10°C. After the dropwise addition, react at room temperature until no raw material remains as detected by TLC (ninhydrin color development). Aqueous citric acid (10%) was added to the reaction liquid to wash three times, the organic phase was spin-dried, the residue was dissolved in dichloromethane, and washed three times with water. The organic phase was dried over...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com