Preparation method of carboxymethyl chitin critical hydrogel
A carboxymethyl chitin and hydrogel technology, applied in pharmaceutical formulations, medical science, surgery, etc., can solve problems such as difficult terminal sterilization, slowing down, etc., achieve long-term analgesic effect, reduce safety hazards, and reduce Painful effects of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
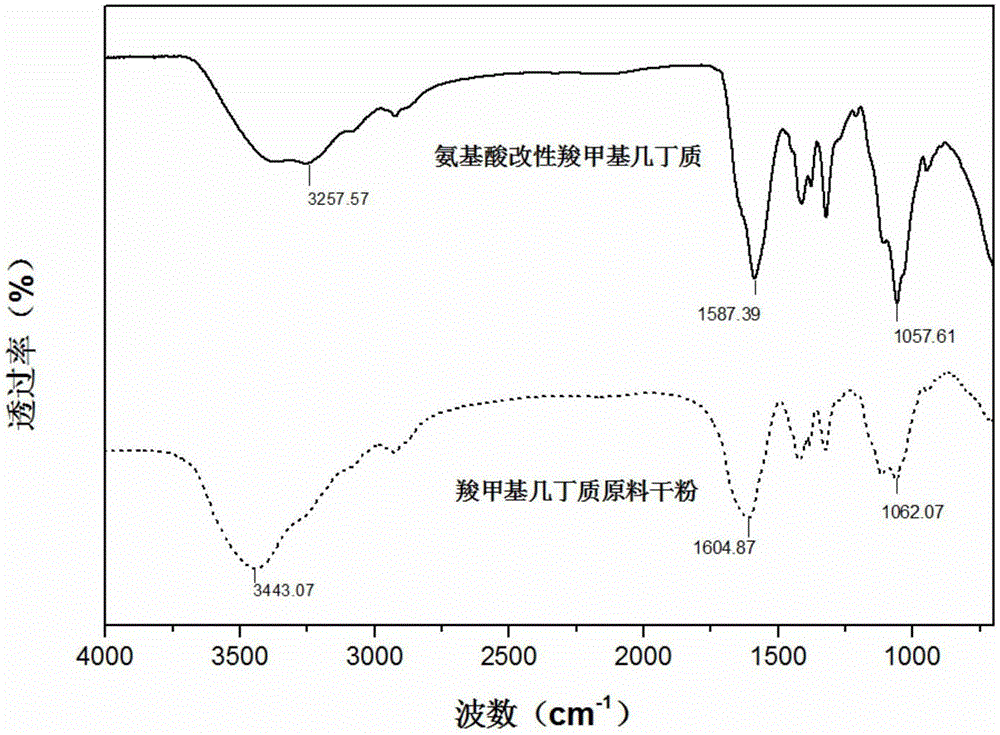
Image
Examples
Embodiment 1
[0028] Dissolve 2g of carboxymethyl chitin dry powder (molecular weight: 580,000 Daltons, degree of deacetylation: 13.41%) in 50mL of water for injection, stir well, then add 0.5g of lysine and 1g of 4-(4,6- Dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride. After mixing, the pH of the reaction solution was adjusted to 6.0 with 1M HCl, and reacted at 37° C. for 24 hours.
[0029] Homogenize the reaction mixture, slowly add 2 times the volume of ethanol dropwise under stirring, wash the dry powder twice with ethanol, and dry it in vacuum for 2 hours, then dissolve it in 100mL of water for injection. Stir well and adjust the pH value to 7.2 with 1M HCl to obtain a transparent and uniform critical state gel. And canned in a disposable glass syringe, sterilized at 121°C for 15 minutes to obtain a carboxymethyl chitin critical state hydrogel that can be used for intra-articular injection.
Embodiment 2
[0031]Dissolve 1.5g of carboxymethyl chitin dry powder (molecular weight: 650,000 Daltons, degree of deacetylation: 9.95%) in 60mL of water for injection, stir well, then add 0.8g of lysine and 2.5g of 4-(4, 6-dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride. After mixing, the pH of the reaction solution was adjusted to 7.5 with 1M HCl, and reacted at 25° C. for 48 hours.
[0032] The mixture obtained by the reaction was homogenized, and slowly added dropwise 2 times the volume of ethanol in the mixture under stirring, and the dry powder was washed with ethanol twice, dried in vacuum for 4 hours, and then dissolved in 90 mL of water for injection. Stir well and adjust the pH value to 6.5 with 1M HCl to obtain a transparent and uniform critical state gel. And canned in a disposable glass syringe, sterilized at 121° C. for 15 minutes to obtain carboxymethyl chitin critical state hydrogel that can be used for intra-articular injection.
Embodiment 3
[0034] Dissolve 5 g of carboxymethyl chitin dry powder (molecular weight: 840,000 Daltons, degree of deacetylation: 7.99%) in 75 mL of water for injection, stir well, then add 1.5 g of lysine and 0.25 g of 4-(4,6 -dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride. After mixing, the pH value of the reaction solution was adjusted to 7.2 with 1M HCl, and placed at 4° C. for 240 h.
[0035] The mixture obtained by the reaction was homogenized, and slowly added dropwise 3 times the volume of ethanol of the mixture under stirring, the dry powder was washed with ethanol 5 times, dried in vacuum for 30min, and then dissolved in 200mL of water for injection. Stir thoroughly and evenly, and adjust the pH value to 7.0 with 1M HCl to obtain a transparent and uniform critical state gel. And canned in a disposable glass syringe, sterilized at 121° C. for 15 minutes to obtain carboxymethyl chitin critical state hydrogel that can be used for intra-articular injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com