Preparation method for triple inactivated vaccine of recombinant Newcastle disease, bird flu and infectious bronchitis
A technology for bronchitis and Newcastle disease, applied in the field of vaccines, can solve problems such as lack of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 (construction of recombinant chicken Newcastle disease virus)
[0026] 1. Construction of auxiliary plasmid NP, P, L genes
[0027] The three genes NP, P, and L were amplified by RT-PCR, and then the three genes were cloned into the expression plasmid vector pTM1, and the constructed helper plasmid was identified by DNA sequence analysis, and the positive plasmid was named pTM-NP, pTM-P, pTM-L.
[0028] 2. Construction of recombinant Newcastle disease virus expressing S1 protein
[0029] Linearize the TOPO TA Cloning vector through a pair of specific primers pM-s and pM-r, use the IBV-M41 strain as a template, and amplify the S1 gene with a pair of specific primers S1-F and S1-R to obtain rLaSota / S1 recombinant subclone. Finally, the rLaSota / S1 subclone was linearized with the In-fusion PCR Cloning Kit and connected to the full-length LaSota cDNA plasmid, which was transformed into Stbl2 competent cells, cultured at 30°C for 24 hours, and then identified...
Embodiment 2
[0044] Embodiment 2 (propagation of recombinant Newcastle disease virus and preparation of triple inactivated vaccine)
[0045] 1. Virus reproduction
[0046] The chicken embryos are selected from the eggs produced by healthy chicken flocks with good feeding and management, and each batch of chicken embryos must be strictly screened. A total of 4 items of external inspection: white shell, size, damage and dirty embryos, a total of 9 items of internal inspection: removal of sand shells, partial air chambers, free air chambers, inverted embryos, sterile eggs, terminated embryos, weak embryos, polluted embryos, Crack embryo. Qualified chicken embryos can be used as seedling making materials. Dilute recombinant Newcastle disease virus rLaSota / S1-HA strain 1:8000 times, inoculate 10-day-old SPF chicken embryos in the allantoic cavity, 0.1 mL per embryo, set the relative humidity at 60% to 65%, and the temperature at 33 to 35°C Incubate in the lower position without turning the e...
Embodiment 3
[0063] Embodiment 3 (immune effect of recombinant Newcastle disease, bird flu, infectious bronchitis triple inactivated vaccine)
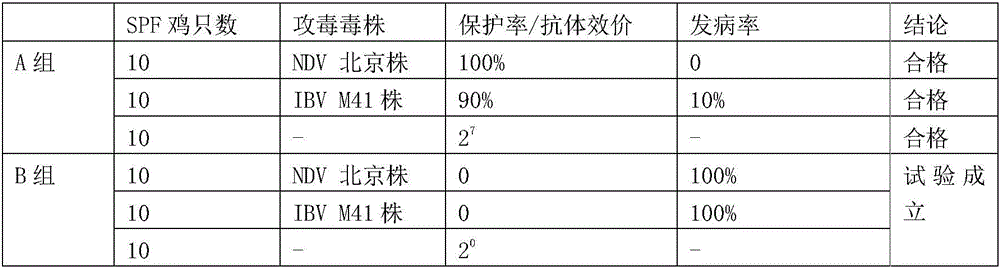
[0064] Sixty 21-day-old SPF chickens were randomly divided into two groups, of which 30 in group A were inoculated with recombinant chicken Newcastle disease, avian influenza and infectious bronchitis triple inactivated vaccine group, and 30 in group B were blank control group. 14 days after immunization, 1ml of Beijing strain (including 10 4 ELD50), infectious bronchitis M41 strain challenge dose is 10 4.0 EID 50 , Measuring AIV-HI antibody titer. The challenge group was observed for 10 days to observe its morbidity and death. Only the antibody titer was determined in the blood collection group. The specific results are shown in Table 3:
[0065] Table 3 Experimental results of the immune effectiveness of the dual vaccine
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com