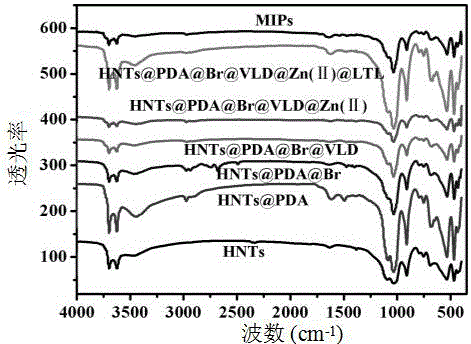
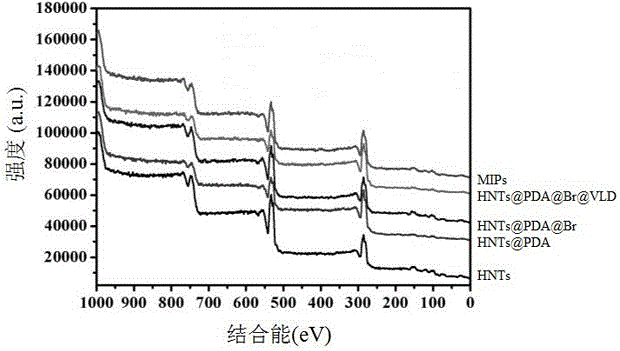
Preparation method of halloysite surface initiated boron affinity imprinted polymer adsorbent
An imprinted polymer, surface-initiated technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of inability to quickly identify and separate luteolin, poor regeneration performance, etc., and achieve good chemical stability and rapid adsorption of materials. Effects of kinetics, good specific adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Preparation of vinyl fluorophenylboronic acid (F-BA):
[0050] In an ice-water bath, add 2 g of fluorophenylboronic acid (4-(aminoethylcarbamoyl)-3-fluorophenylboronic acid) to 100 mL of sodium carbonate solution, then add 3 mL of acryloyl chloride to the above solution, and then add 0.1 mol / L of sodium hydroxide The pH of the solution was adjusted to neutrality for 12 hours, and finally vacuum-dried at 60°C.
[0051] (2) Activation of halloysite nanotubes (HNTs):
[0052] Grinding and pulverizing 0.1g block halloysite nanotubes through 100 sieves, calcining at 100°C for 24h,
[0053] The calcined 0.1g of HNTs was placed in 50mL of a mixed solution of sulfuric acid and hydrochloric acid (v:v, 1:3), and stirred at 80°C for 6h; Dry at ℃.
[0054] (3) Preparation of dopamine loaded on halloysite nanotubes (HNTs@PDA):
[0055] 100mg of activated halloysite nanotubes were put into 40mL of ethanol and water solution (v:v, 1:1), and then 50mg of dopamine hydrochloride ...
Embodiment 2
[0072] (1) Preparation of vinyl fluorophenylboronic acid (F-BA):
[0073] In an ice-water bath, add 3 g of fluorophenylboronic acid (4-(aminoethylcarbamoyl)-3-fluorophenylboronic acid) to 150 mL of sodium carbonate solution, then add 4 mL of acryloyl chloride to the above solution, and then use 0.1 mol / L of sodium hydroxide solution Adjust the pH to neutral and react for 12 hours, and finally dry it under vacuum at 60°C.
[0074] (2) Activation of halloysite nanotubes (HNTs):
[0075] 0.2g block halloysite nanotubes were ground and crushed through 100 sieves, and calcined at a high temperature of 100°C for 24h. The calcined 0.2g HNTs were placed in a mixed solution of 60mL sulfuric acid and hydrochloric acid, (v:v, 1 :3) Stir at 80°C for 6h. Then rinse with a large amount of distilled water until neutral, and finally dry it under vacuum at 60°C.
[0076] (3) Preparation of dopamine loaded on halloysite nanotubes (HNTs@PDA):
[0077] Put 150mg of activated halloysite nanotube...
Embodiment 3
[0089] (1) Preparation of vinyl fluorophenylboronic acid (F-BA):
[0090] In an ice-water bath, add 4 g of fluorophenylboronic acid (4-(aminoethylcarbamoyl)-3-fluorophenylboronic acid) to 200 mL of sodium carbonate solution, then add 5 mL of acryloyl chloride to the above solution, and then add 0.1 mol / L of sodium hydroxide The pH of the solution was adjusted to neutrality for 12 hours, and finally vacuum-dried at 60°C.
[0091] (2) Activation of halloysite nanotubes (HNTs):
[0092] Grind and crush 0.3g massive halloysite nanotubes through a 100 sieve, and calcined at 100°C for 24h. The calcined 0.3g of HNTs was placed in 70mL of a mixed solution of sulfuric acid and hydrochloric acid (v:v, 1:3 ), stirred at 80°C for 6h. Then rinse with a large amount of distilled water until neutral, and finally dry it under vacuum at 60°C.
[0093] (3) Preparation of dopamine loaded on halloysite nanotubes (HNTs@PDA):
[0094] 200mg of activated halloysite nanotubes were put into 60mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com