Staphylococcus drug sensitive strip and preparation method thereof
A staphylococcus, slat technology, applied in biochemical equipment and methods, microorganism-based methods, biochemical instruments, etc., can solve the problem of few types of antibiotics, the design of antibiotic concentration cannot reflect the latest clinical drug information, and the type of antibiotics cannot be adapted to clinical strains drug resistance and other issues, to achieve great application value and reduce the effect of breeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Production of staphylococcal drug-sensitive lath products:
[0050] (1) Configure carnosol aqueous solution
[0051] Weigh 0.4g carnosol and dissolve it in 2000mL distilled water.
[0052] (2) Sterilize the prepared carnosol aqueous solution at 121°C for 15 minutes, and then distribute it into 96 sterilized test tubes, each with 15 mL.
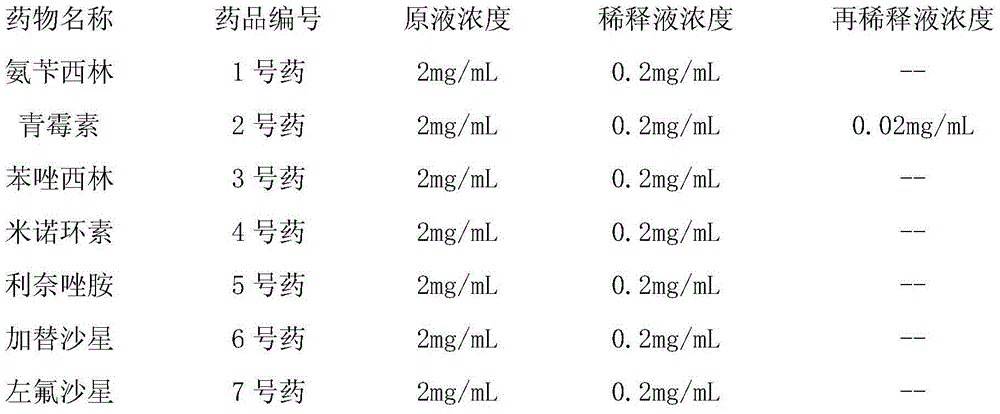
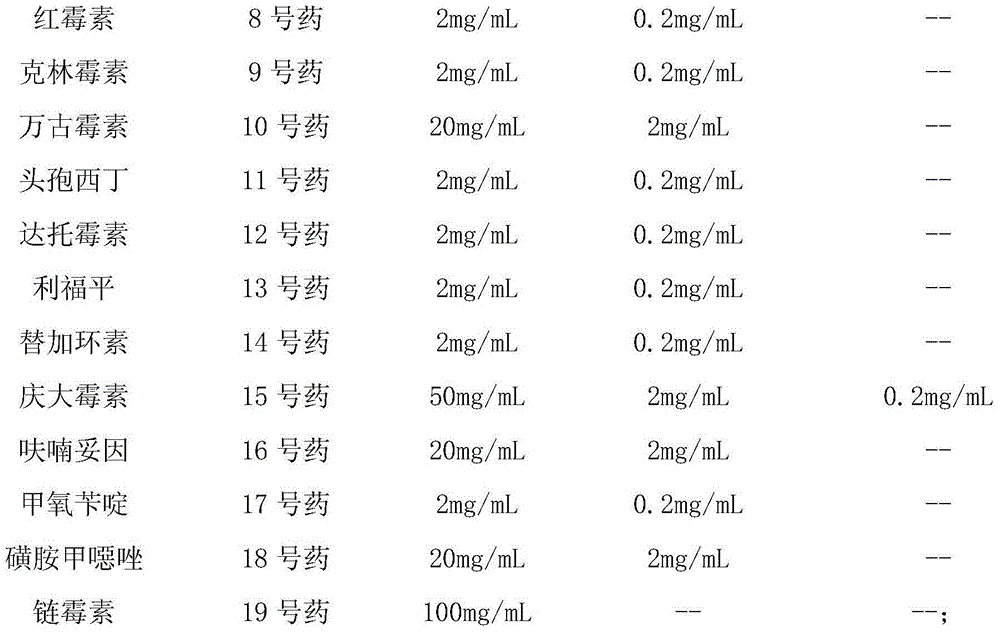
[0053] (3) Prepare each antibiotic stock solution.
[0054] Prepare the stock solution of each antibiotic according to the concentration in the table below:
[0055]
[0056] (4) Configure each well of antibiotic solution
[0057] According to the following requirements, add the antibiotic stock or dilution into the 15 mL test tube containing carnosol aqueous solution to be used.
[0058]
[0059]
[0060]
[0061]
[0062]
[0063] (5) Add sample and split slats
[0064] Load the configured 96 test tubes on the sampler, use the sampler to add samples to the designated staphylococcal drug sensitivity plate, add 50μL to each well;
[0065] (6) Put the ...
Embodiment 2
[0070] The performance test of staphylococcal drug susceptibility board comes from CLSI's broth dilution method.
[0071] (1) Strain recovery
[0072] Kaikai quality control strain Staphylococcus aureus (ATCC 29213) (purchased from Beijing Baote Medical Equipment Co., Ltd. ATCC29213 first generation strain), made a bacterial suspension with 0.9% saline, and inoculated an appropriate amount into nutrient agar medium Incubate at 35°C for 18-24 hours.
[0073] (2) Subculture
[0074] Take an appropriate amount of the resuscitated culture to inoculate the nutrient agar medium slant and incubate at 35°C for 20 hours.
[0075] (3) Strain enrichment culture
[0076] Inoculate the above-mentioned fresh culture after passage to nutrient agar medium and cultivate at 35°C for 20 hours.
[0077] (4) Preparation of bacterial suspension
[0078] The fresh culture of Staphylococcus aureus after the above-mentioned enrichment culture is diluted with 0.9% sterile sodium chloride solution, and after turbid...
Embodiment 3
[0091] Staphylococcal drug sensitivity plate performance test
[0092] (1) Strain recovery
[0093] Open the quality control strain Enterococcus faecalis (ATCC 29212) (same as above), make a bacterial suspension with 0.9% saline, and inoculate an appropriate amount into nutrient agar medium, cultivate at 35°C for 18-24 hours.
[0094] (2) Subculture
[0095] An appropriate amount of the resuscitated culture was inoculated onto the nutrient agar medium slope, and cultivated at 35°C for 22 hours.
[0096] (3) Strain enrichment culture
[0097] Inoculate the above-mentioned fresh culture after passage to nutrient agar medium and cultivate at 35°C for 22 hours.
[0098] (4) Preparation of bacterial suspension
[0099] The fresh culture of Enterococcus faecalis after the above-mentioned enrichment culture is diluted with 0.9% sterile sodium chloride solution, and after turbidity, it is prepared into a bacterial suspension with a turbidity of 0.5 McDonnell standard.
[0100] (5) Broth dilution
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com