Gene-carrying compound for multiple targeting modification, preparation method and application
A technology of targeted modification and compound, applied in other methods of inserting foreign genetic materials, gene therapy, medical preparations of non-active ingredients, etc., can solve the problem of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation method of the polycationic gene carrier (I) of membrane-targeting peptide modification, comprises the following steps:
[0058] Under nitrogen protection, polyethyleneimine-poly(glycolide-co-caprolactone)-polyethyleneimine copolymer with a weight average molecular weight of 30000, said polyethyleneimine-poly(glycolide- (co-caprolactone)-polyethyleneimine copolymer has the same weight-average molecular weight of branched polyethyleneimine, and is 10000, and the two ends with weight-average molecular weight of 2000 are respectively adjacent dithiopyridyl and succinimide-modified polyethylene glycol (OPSS-PEG-NHS), dissolved in a mixed solvent, reacted for 3 hours at room temperature and protected from light, added membrane targeting peptide, reacted for 6 hours, and the product was dissolved in distilled water Dialyze for 48h, change the distilled water every 2h, and freeze-dry to obtain the polycationic gene carrier (I) modified by the membr...
Embodiment 2
[0075] Embodiment 2: Preparation of REDV-NP, pGFP / REDV-NP complex and TAT-NLS / pGFP / REDV-NP complex:
[0076] (1) Dissolve the abbreviated TAT-NLS polypeptide in an appropriate amount of water at room temperature (the amount of water can dissolve the TAT-NLS polypeptide);
[0077] Nucleic acid (the nucleic acid used in this embodiment is a plasmid containing green fluorescent protein) is dissolved in an appropriate amount of water, (the amount of water can dissolve the plasmid of green fluorescent protein);
[0078] According to the mass ratio of TAT-NLS polypeptide and nucleic acid in abbreviation as 1:1, mix the TAT-NLS polypeptide aqueous solution and nucleic acid aqueous solution evenly, and let it stand for 30 minutes; obtain the mixed solution;
[0079] (2) Prepare the polycationic gene carrier (I) (REDV-NP) nanoparticle suspension modified by the membrane-targeting peptide by dialysis:
[0080] Weigh 5 mg of membrane-targeting peptide-modified polycationic gene carrier ...
Embodiment 3
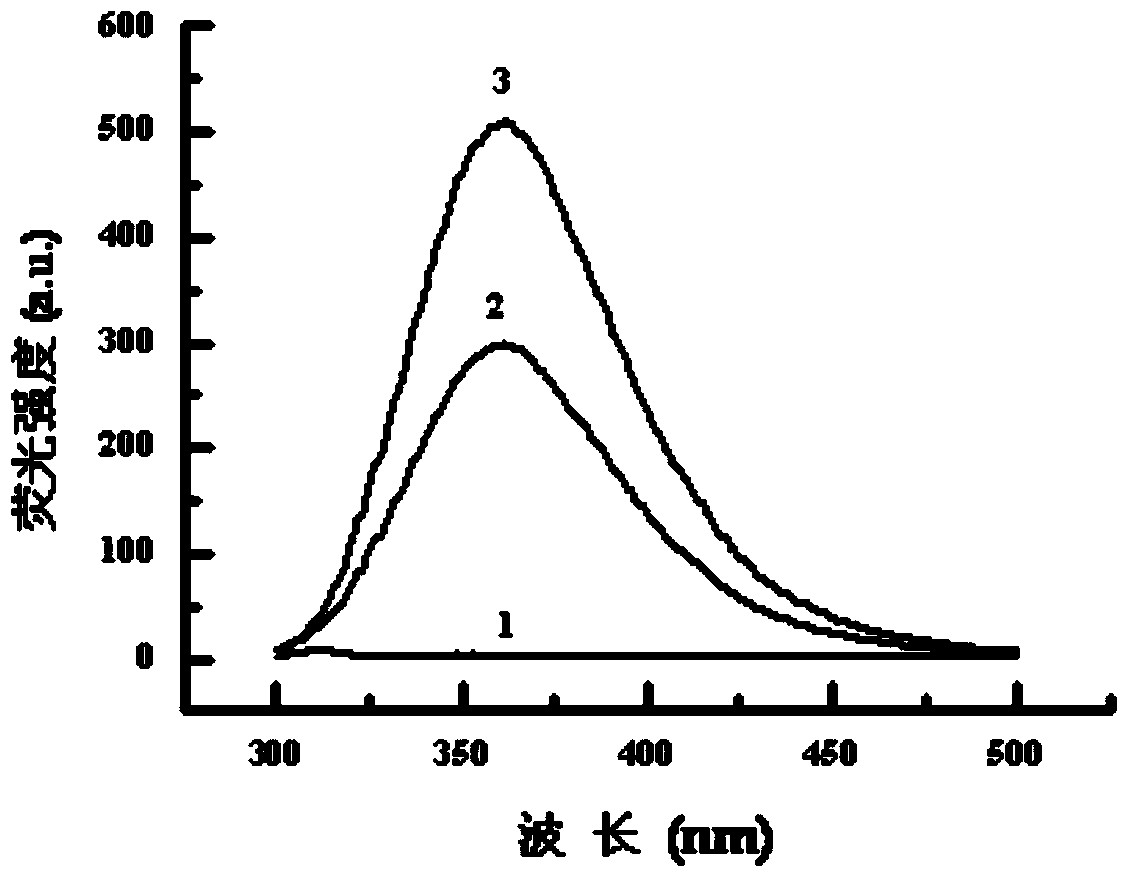
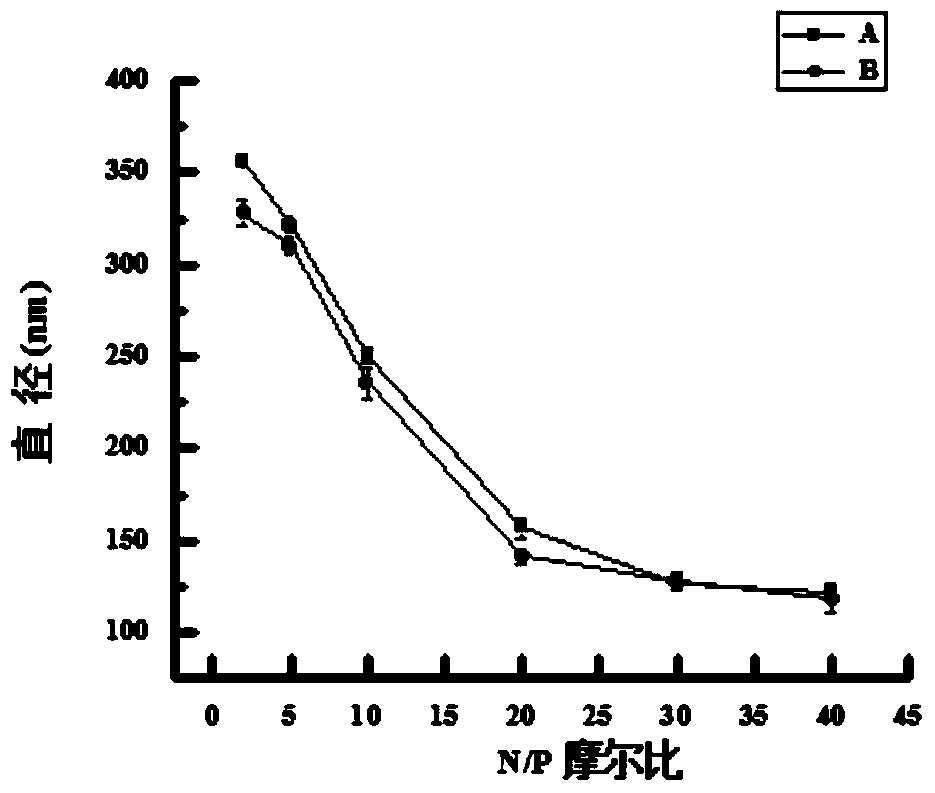
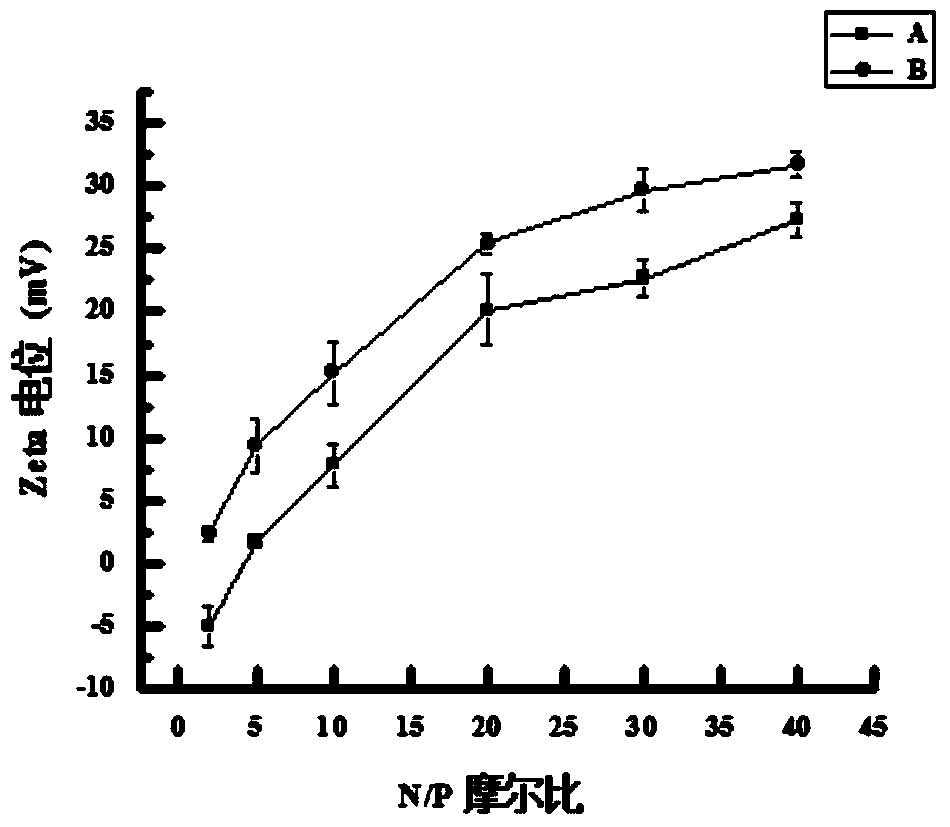
[0089] Embodiment 3: the agarose gel electrophoresis analysis of TAT-NLS / pGFP / REDV-NP and pGFP / REDV-NP:
[0090] Add the prepared TAT-NLS / pGFP / REDV-NP, pGFP / REDV-NP and pure pGFP genes with different N / P ratios to the wells of 0.8% agarose gel respectively, and place them in 1×TAE buffer at 100V Electrophoresis in liquid for 30min. Observe the position of pGFP under ultraviolet irradiation and take pictures to analyze the binding ability of nanoparticles and pGFP. From Figure 4 As can be seen in , REDV-NP can fully compress loaded pGFP at N / P ratio ≥ 20. After adding TAT-NLS, TAT-NLS / pGFP / REDV-NP can completely block the migration of plasmids when the N / P ratio is ≥10, which indicates that TAT-NLS helps REDV-NP nanoparticles to bind better and Compress pGFP.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com