Citrus tristeza virus based vectors for foreign gene/s expression
A virus vector, gene cassette technology, applied in genetic engineering, plant genetic improvement, application, etc., can solve the problem of low stability of foreign inserts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Protoplast preparation, transfection, RNA isolation and Northern Blot analysis
[0077]Tobacco benthamiana leaf protoplasts were prepared according to the method invented by Nava-Castillo et al., (1997). Disinfect the surface of Bensheng tobacco leaves for 3 weeks, use a sterile knife to lightly scratch the lower surface, and culture overnight in a dark room (16-20 hours), the medium is 0.7M MMC (0.7M mannitol, 5mM MES, 10mM CaCl 2 ) plus 1% cellulose (Yakult Honsh, Tokyo, Japan) and 0.5% polygalacturonase (Calbiochem Laboratories, La Jolla, California).
[0078] Use Sp6 RNA polymerase (Wisconsin State Epicentre Technologies Laboratory) to obtain capped in vitro RNA (Satyanarayana et al., 1999) on NotI or StuI linear plasmid DNA, use PEG (polyethylene glycol) to transfect protoplasts, this method refers to Satyanarayana et al., (1999). Four days after transfection, protoplasts were used to prepare total RNA for Northern blot analysis, and virions were isolated. Aliqu...
Embodiment 1
[0087] Example 1: System for detecting CTV-based expression vectors
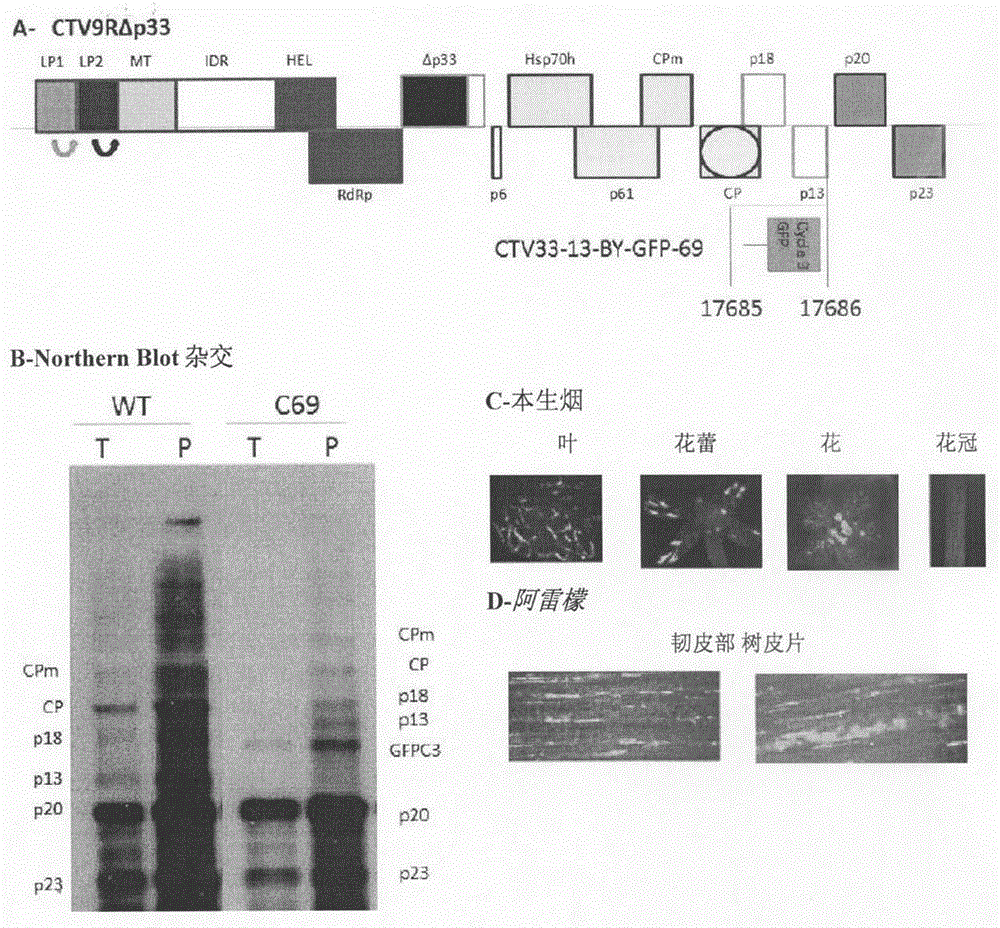
[0088] CTV-based expression vectors were tested in three systems, respectively, N. benthamiana leaf pulp protoplasts, N. benthamiana whole plant, and Alemon whole plant. Vectors for infecting whole plants were constructed using a full-length CTV cDNA clone (pCTV9R) and a mostly deleted mutant of the p33 gene (pCTV9RΔp33), which has a PstI restriction site deleted, is easier to clone, and retains the ability to infect most citrus plants. Competence (Tatineni et al., 2008). Faster analysis of N. benthamiana protoplasts requires construction of a vector on the SP6 transcriptional plasmid (Satyanarayana et al., 1999). In protoplasts it is convenient to use the minireplicon pCTVΔCla333R (Gowda et al., 2001) with most 3' gene deletions. The overall goal is to obtain citrus trees infected with different CTV expression vectors, which is more difficult and time-consuming. Soil inoculation of citrus trees has been ...
Embodiment 2
[0089] Example 2: Genes added at different positions in the CTV genome
[0090] Insertion at the p13 gene locus
[0091] Some researchers (Folimonov et al., 2007) inserted other genes between the two capsid protein genes in an effective CTV vector, making the exogenous gene the sixth gene starting from the 3' end. But the CTV gene with the highest expression tends to be near the 3' end. Inserting a gene close to the 3' end will therefore result in increased expression. P13 is the third gene counted from the 3' end, and it is a relatively high expression gene, which is not required for infection of most CTV hosts (Tatineni et al., 2008; Tatineni et al., in preparation). But previous attempts to replace p13ORF with GFP ORF failed (Folimonov et al., 2007). There are several possibilities for the cause of failure. Previously the vector was designed with the assumption that translation starts at the first start codon, but the p13ORF has a second in-frame AUG. Transcription may...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com