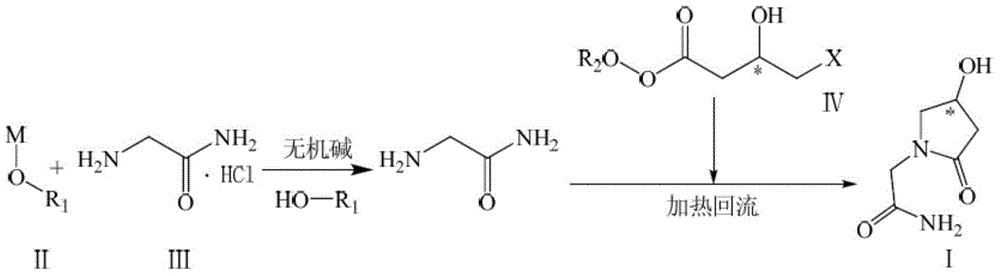
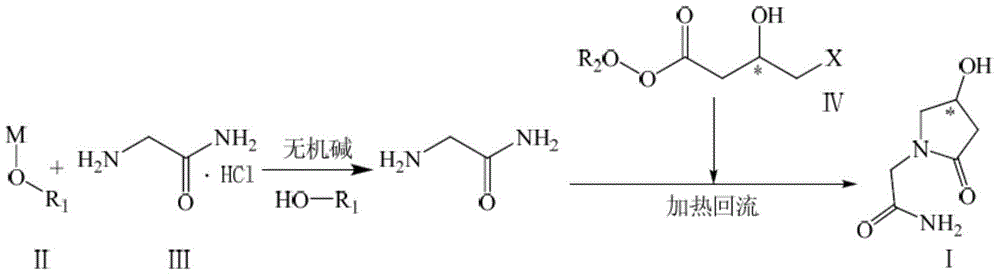
Preparation method of optically active pure 1-(carbamoyl)methyl-4-hydroxy-2-pyrrolidone
A carbamoyl and pyrrolidone technology, applied in the field of drug synthesis, can solve the problems of low solubility of glycinamide hydrochloride, difficult to remove subsequent residues, troublesome product post-processing, etc., and achieves improved product purity, improved tailing, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of (S)-1-(carbamoyl)methyl-4-hydroxyl-2-pyrrolidone
[0032] Add sodium ethoxide / dehydrated ethanol solution [30.62g (0.45mol) / 500ml] into the three-necked flask, add 5.30g (0.05mol) of anhydrous sodium carbonate and 55.25g (0.50mol) of glycinamide hydrochloride, and stir at room temperature Evenly, heat up to reflux, keep reflux for 1h, add anhydrous sodium carbonate 53.00g (0.50mol) for the second time, stir and slowly add (S)-4-chloro-3-hydroxy-butyric acid ethyl ester 91.68g (0.55mol ), keep the reflux for about 20 hours.
[0033] Stop the reaction, filter out the inorganic salt while hot, and concentrate under reduced pressure to obtain a reddish-brown oily substance; add water to dissolve, wash with dichloromethane, and concentrate the aqueous phase under reduced pressure to obtain a reddish-brown oily substance;
[0034] Silica gel column chromatography, dichloromethane / methanol / ethanol system gradient elution (V / V / V=30 / 1~4 / 0~1), combin...
Embodiment 2
[0038] Example 2: Preparation of (R)-1-(carbamoyl)methyl-4-hydroxyl-2-pyrrolidone
[0039] Add potassium ethylate / absolute ethanol solution [29.45g (0.35mol) / 500ml] in the three-necked flask, add anhydrous potassium carbonate 20.73g (0.15mol) and glycinamide hydrochloride 55.25g (0.50mol), and stir at room temperature Evenly, heat up to reflux, keep reflux for 1h, add anhydrous potassium carbonate 103.65g (0.75mol) for the second time, stir and slowly add (R)-4-chloro-3-hydroxy-butyric acid ethyl ester 91.68g (0.55mol ), keep the reflux for about 20 hours.
[0040] After the reaction was completed, post-treatment was carried out in the same manner as in Example 1 to obtain 56.12 g of (R)-1-(carbamoyl)methyl-4-hydroxy-2-pyrrolidone, with a yield of 71.04%.
Embodiment 3
[0041] Example 3: Preparation of (S)-1-(carbamoyl)methyl-4-hydroxyl-2-pyrrolidone
[0042] Add sodium methoxide / anhydrous methanol solution [13.51g (0.25mol) / 500ml] into the three-necked flask, add 26.50g (0.25mol) of anhydrous sodium carbonate and 55.25g (0.50mol) of glycinamide hydrochloride, and stir at room temperature Evenly, heat up to reflux, keep reflux for 1h, add anhydrous sodium carbonate 66.25g (0.625mol) for the second time, stir and slowly add (S)-4-chloro-3-hydroxy-butyric acid ethyl ester 91.68g (0.55mol ), keep the reflux for about 26 hours.
[0043] After the reaction was completed, post-treatment was carried out in the same manner as in Example 1 to obtain 53.01 g of (S)-1-(carbamoyl)methyl-4-hydroxy-2-pyrrolidone, with a yield of 67.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com