Pharmaceutical composition for treating glioma
A technology for glioma and composition, applied in the field of medicine, can solve the problems of small selection of drugs, lack of effective drugs, etc., and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
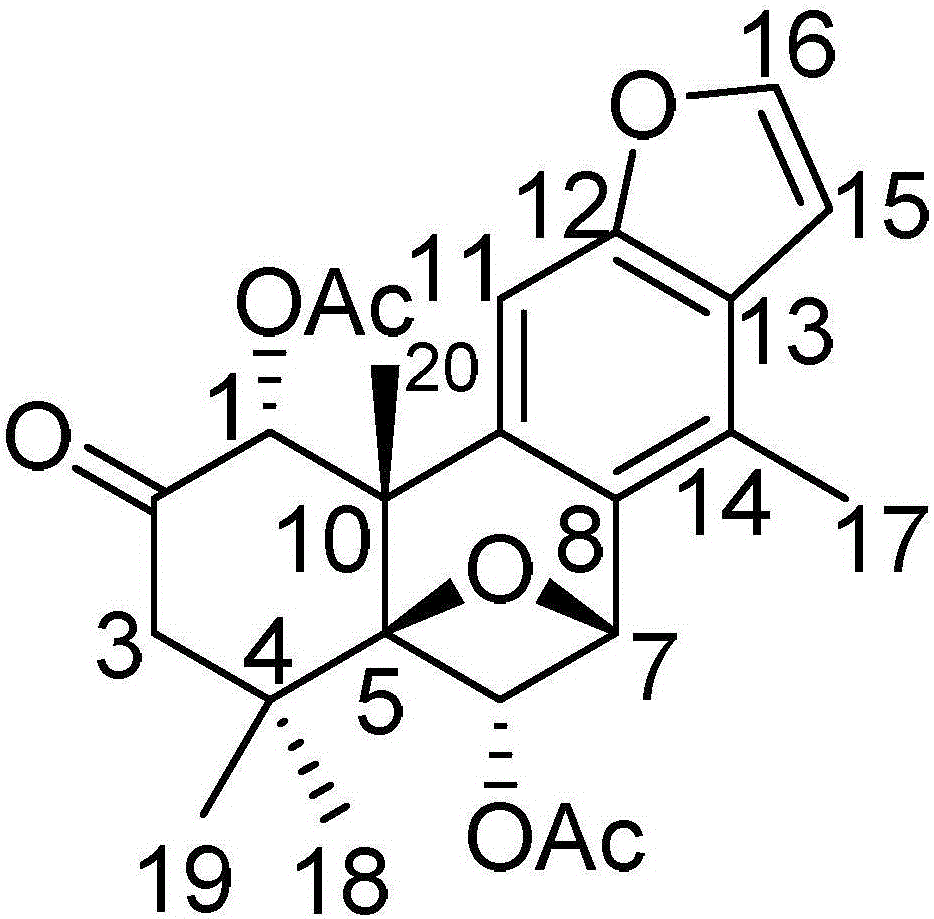
[0043] Embodiment 1: A kind of pharmaceutical composition for treating glioma, according to percentage by weight, it is made up of 55% component A, 23% component B, 10% component C and 12% component D, group Part A is a diterpene compound (I) having the following structural formula:
[0044]
[0045] Component B is withanolide compound (II) having the following structural formula:
[0046]
[0047] Component C is a triterpenoid saponin compound echinosideA, structural formula (Ⅲ):
[0048]
[0049] Component D is a flavonoid compound (IV) having the following structural formula:
[0050]
[0051] Wherein, the separation preparation and structural confirmation of the diterpene compound (I) in component A are as follows:
[0052] Main materials, sources of reagents and types of instruments:
[0053] Ethanol, petroleum ether, ethyl acetate, n-butanol, and dichloromethane were of analytical grade, purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Methanol,...
Embodiment 2
[0070] Embodiment 2: the pharmaceutical composition pharmacological action test in embodiment 1
[0071] 1. Materials and Instruments
[0072] In this experiment, the human brain malignant glioblastoma U251 cell line was purchased from the American Type Culture Collection. DMEM high-glucose medium was purchased from Hyclone Company of the United States; sodium chloride, sodium hydroxide, potassium chloride, oxygen chloride, sodium hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, potassium dihydrogen phosphate, and methanol were purchased from Nanjing chemical plant. Glycine, Tris, Tween20, and dimethyl sulfoxide (DMEM) were purchased from Sigma, USA; Aimexin-V / PI cell death kit was purchased from Invitrogen, USA; cell lysate, 0.25% trypsin, CellCountingKit-8 kit , penicillin, streptomycin, Hoechst33258, nuclear dye DAPI, PMSF, Westernblot gel preparation kit, nuclear protein extraction kit, Coomassie brilliant blue staining solution, standard protein, 5X ...
Embodiment 3
[0096] A pharmaceutical composition for treating glioma, comprising a therapeutically effective amount of the pharmaceutical composition of Example 1 above and a pharmaceutically acceptable carrier. The pharmaceutically acceptable carrier is selected from one or more of the following: lactose, sucrose, gelatin, agar, pectin, gum arabic, magnesium stearate, stearic acid, lower alkyl ethers of cellulose , Corn Starch, Potato Starch, Gums, Fatty Acids, Glyceryl Monostearate. The daily effective dosage of the pharmaceutical composition (excluding pharmaceutically acceptable carrier) in the above-mentioned Example 1 is 0.001-0.006g / kg human body, and it can be made into the medicine with corresponding content according to the need, and taken according to the doctor's advice.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com