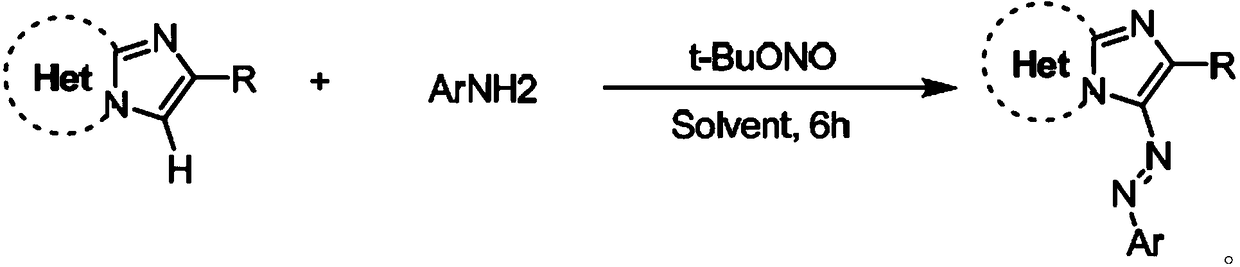
A kind of imidazoheterocyclic azo derivative and its preparation method and application
A technology of heterocyclic azo derivatives, applied in the field of imidazo heterocyclic azo derivatives and its preparation, can solve the problems of cumbersome coupling reaction process, lower the decomposition temperature of diazonium salt, and difficult hydrolysis, etc., and achieve excellent fluorescence effect of nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
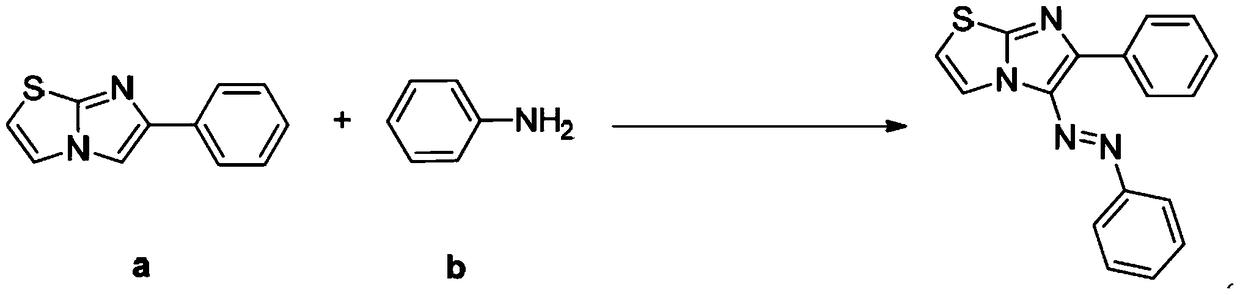
[0027] Example 1: Synthesis of 6-phenyl-(5-phenylazo) imidazo[2,1-b]thiazole
[0028] The synthetic route is:
[0029]
[0030] Specifically include the following steps:
[0031] The compound of formula (a) above, the compound of formula (b) and t-BuONO were adjusted to a molar ratio of 1:1.2:1.5, wherein the compound of formula (a) was 2 mmol. The reaction system was stirred and reacted in 1,2-dichloroethane at 50° C. for 6 hours. Cool after the reaction, filter with short silica gel column, spin evaporate the filtrate, remove the solvent, use silica gel column chromatography for the residue, wash with petroleum ether, TLC detects, combine the effluent containing the product, remove the solvent by rotary evaporator distillation, vacuum Dry to obtain the target product as a red powder, the solution is yellow, the yield is 85%, and the purity is 99.1% (HPLC).
[0032] The effective temperature range of the reaction temperature in the above preparation steps is 40-100°C, t...
Embodiment 2
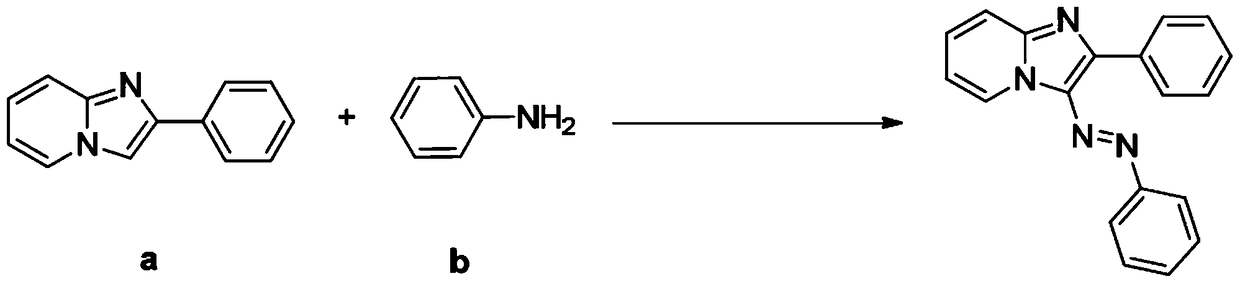
[0038] Example 2: Synthesis of 2-phenyl-(3-phenylazo) imidazo[1,2-a]pyridine
[0039] synthetic route:
[0040]
[0041] Specifically include the following steps:
[0042] The compound of formula (a) above, the compound of formula (b) and t-BuONO were adjusted to a molar ratio of 1:1.2:1.5, wherein the compound of formula (a) was 2 mmol. The reaction system was stirred and reacted in 1,2-dichloroethane at 50° C. for 6 hours. Cool after the reaction, filter with short silica gel column, spin evaporate the filtrate, remove the solvent, use silica gel column chromatography for the residue, wash with petroleum ether, TLC detects, combine the effluent containing the product, remove the solvent by rotary evaporator distillation, vacuum Drying gave the target product as a yellow powder with a yield of 60% and a purity of 99.3% (HPLC).
[0043] The effective temperature range of the reaction temperature in the above preparation steps is 40-100°C, the yield at 40°C is 51%; the yi...
Embodiment 3
[0049] Example 3: Synthesis of 2-(5-phenylazo)imidazo[2,1-b]thiazol-6-yl-phenol
[0050] The synthetic route is:
[0051]
[0052] Specifically include the following steps:
[0053] The compound of formula (a) above, the compound of formula (b) and t-BuONO were adjusted to a molar ratio of 1:1.2:1.5, wherein the compound of formula (a) was 2 mmol. The reaction system was stirred and reacted in 1,2-dichloroethane at 50° C. for 6 hours. Cool after the reaction, filter with short silica gel column, spin evaporate the filtrate, remove the solvent, use silica gel column chromatography for the residue, wash with petroleum ether, TLC detects, combine the effluent containing the product, remove the solvent by rotary evaporator distillation, vacuum Drying gave the target product as a yellow liquid in 76% yield and 99.3% purity (HPLC).
[0054] The effective temperature range of the reaction temperature in the above preparation steps is 40-100°C, the yield at 40°C is 63%; the yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com