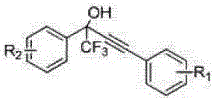
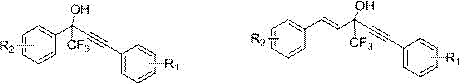A kind of fluorine-containing alkyne alcohol compound and its synthesis method
A synthesis method and compound technology are applied in the field of fluorine-containing alkynol compounds and their novel synthesis, which can solve problems such as environmental pollution, and achieve the effects of simple and convenient operation, environment-friendly synthesis method and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 0.8706 g (5 mmol) of trifluoroacetophenone, 1.65 mL (15 mmol) of phenylacetylene, and 0.0425 g (0.25 mmol Ag + ) as the catalyst, triethylamine 0.14mL (1 mmol), triphenylphosphine 0.0656 g (0.25 mmol), water 20 mL, stirred at 10°C for 15 h. After the reaction was complete, it was extracted with ethyl acetate, and the organic phase was dried and separated by column chromatography to obtain a fluorine-containing alkynol compound (petroleum ether: ethyl acetate = 25:1). The product was a light yellow liquid with a yield of 98%.
[0030] The physicochemical index of this product: 1 H-NMR (400 MHz, CDCl 3 ) : δ=7.71 (s, 2H), 7.40 (d, J=7.6Hz, 2H), 7.30 - 7.29 (m, 3H), 7.26 - 7.19 (m, 3H), 3.43 (s, 1H); 13 C-NMR (CDCl 3 , 100 MHz): 135.5, 132.2, 129.6, 128.6, 128.4, 127.3, 125.0, 122.1,121.1, 88.1, 84.6, 73.6, 73.3; IR (v max / cm -1 ): 3545.8, 3064.7, 2233.7, 1599.7, 1490.9, 1355.1, 1186.4, 1066.7, 933.3, 757.6, 691.0.
Embodiment 2
[0032]Add 0.8706 g (5 mmol) of trifluoroacetophenone, 1.9824 g (15 mmol) of p-methoxyphenylacetylene, 0.0083 g (0.05 mmol) of silver acetate, and 0.21 mL (2 mmol), triphenylphosphine 0.1311 g (0.5 mmol), water 10 mL as solvent, and stirred at 25°C for 15 h. After the reaction was complete, it was extracted with ethyl acetate, and the organic phase was dried and separated by column chromatography to obtain a fluorine-containing alkynol compound (petroleum ether: ethyl acetate = 25:1). The product was a light yellow liquid with a yield of 92%.
[0033] The physicochemical index of this product: 1 H-NMR (400 MHz, CDCl 3 ) : δ=7.81 (d, J=7.6Hz, 2H),7.46 - 7.42 (m, 5H), 6.87 (d, J=8.8Hz, 2H), 3.82 (s, 3H), 3.18 (s, 1H) ; 13 C-NMR (CDCl 3 IR (v max / cm -1 ): 3431.1, 2960.4, 2841.5, 2231.0, 1605.9, 1510.8, 1452.1, 1294.6, 1249.2, 1173.3, 1066.0, 832.3, 707.1.
Embodiment 3
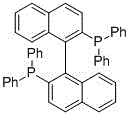
[0035] Under an argon atmosphere, 3.1284 g (15 mmol) of p-chlorotrifluoroacetophenone, 1.8018 g (15 mmol) of p-fluorophenylacetylene, 0.3806 g (3 mmol) of silver fluoride, and 0.73 mL (9 mmol) of pyridine were added to the reaction system. mmol), 0.7923 g (1.5 mmol) of triphenylphosphine substituted by binaphthol derivatives (structural formula (III)), 10 mL of water, heated and stirred at 100°C for 8 h. After the reaction is complete, cool to room temperature, extract with ethyl acetate, and obtain the fluorine-containing alkynol compound (petroleum ether: ethyl acetate=25:1) by column chromatography after the organic phase is dried. The product is a light yellow liquid, and the yield is 98%.
[0036] (III)
[0037] The physicochemical index of this product: 1 H-NMR (400 MHz, CDCl 3 ) : δ=7.77 (d, J=8.4Hz, 2H),7.54 - 7.51 (m, 2H), 7.42 (d, J=8.8Hz, 2H), 7.08 (t, J=8.4Hz, 2H), 3.82 (s,1H); 13 C-NMR (CDCl 3 , 100 MHz): 164.6, 162.1, 135.7, 134.2, 134.1, 133.9,128.7, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com