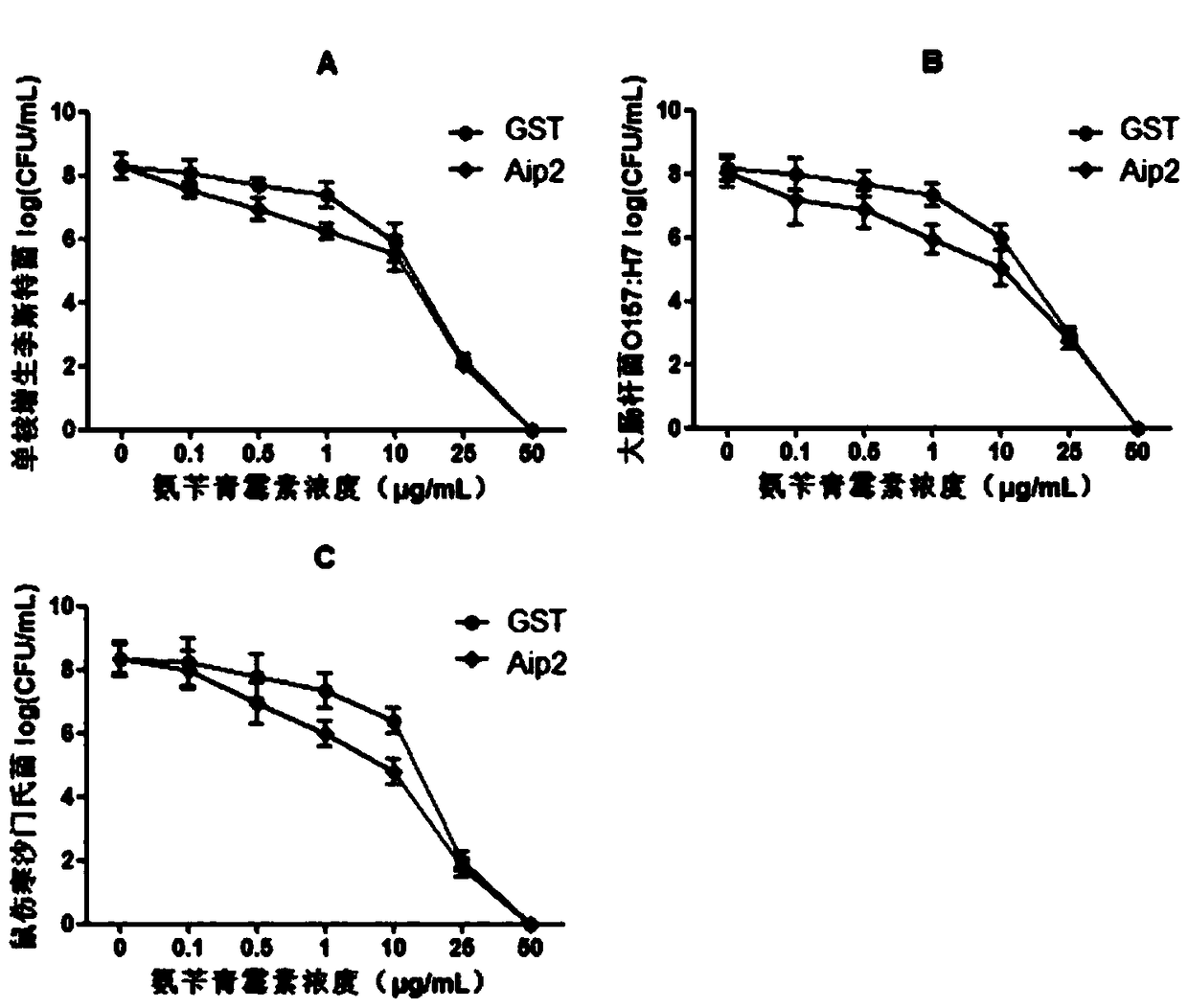
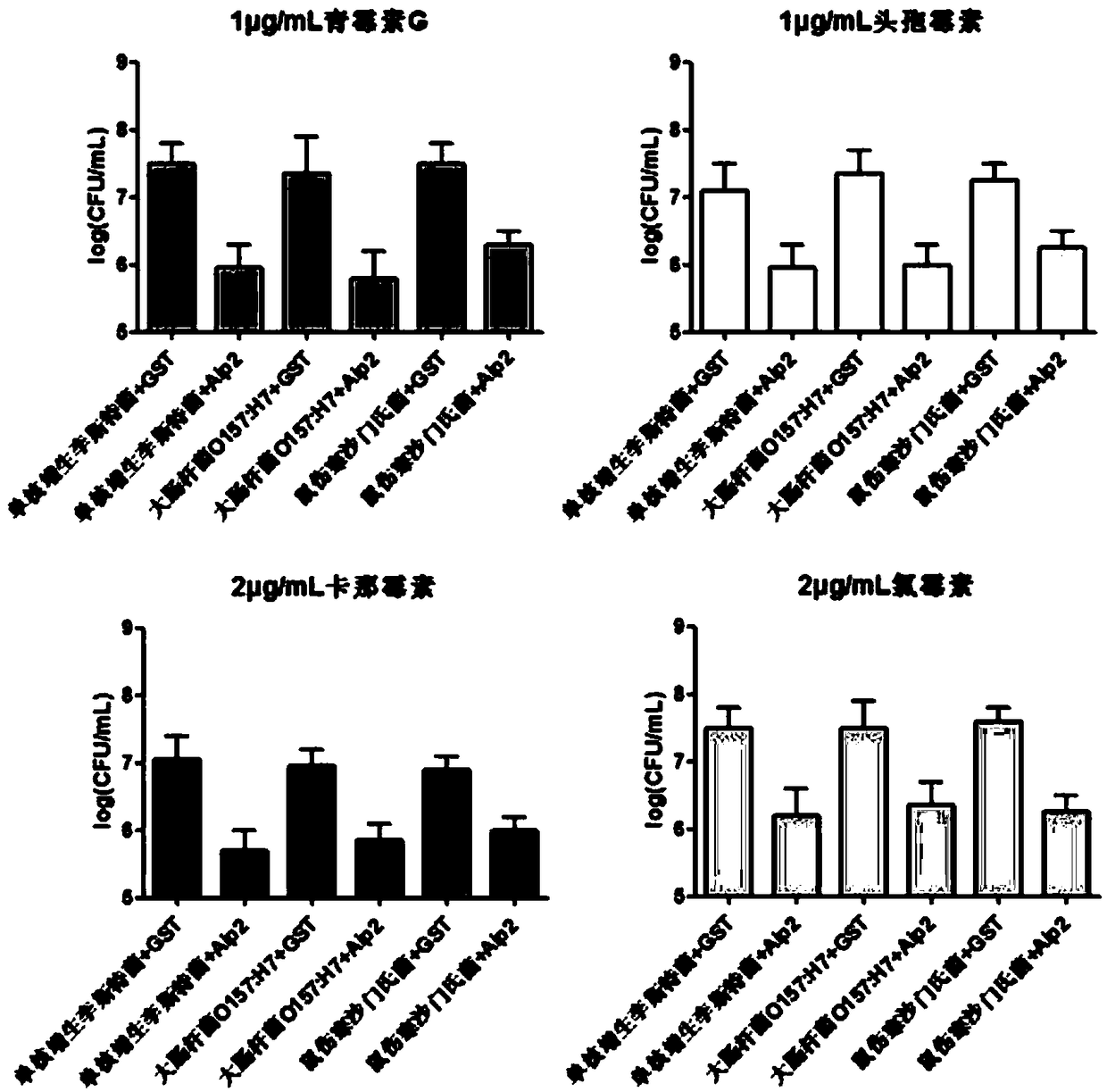
Application of a Bifidobacterium longum protein to enhance the susceptibility of Listeria monocytogenes to antibiotics
A technology of Listeria and antibiotics, applied in the field of microbiology, can solve problems such as the limitation of the application range of Bifidobacterium longum protein, and achieve the effect of improving microbial drug sensitivity, sensitivity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Embodiment 1 (recombinant escherichia coli expression strain that prepares Bifidobacterium longum protein)
[0040] 1.1 Experimental materials
[0041] 1.1.1 Strains and vectors
[0042] Bifidobacterium longum, Escherichia coli DH5α, and vector pGEX-4T-1 were all purchased from the market.
[0043] 1.1.2 Commonly used reagents
[0044] (1) TE buffer (pH 8.0): 10mmol / L Tris-HCl (pH 8.0), 1mmol / L EDTANa 2 (pH8.0). After configuration, autoclave and store at room temperature.
[0045] (2) 10mg / mL lysozyme: 100mg lysozyme dissolved in 10mL ddH 2 O, stored at –20°C for later use.
[0046] (3) 10% SDS: 5g SDS plus ddH 2 O was dissolved and the volume was adjusted to 50 mL.
[0047] (4) 3M KAc: 14.72g KAc dissolved in ddH 2 O and make up to 50mL.
[0048] (5) 3M NaAc: 12.35g NaAc dissolved in ddH 2 O and make up to 50mL.
[0049] (6) 0.1M CaCl 2 : 11.1g CaCl 2 Dissolve in 1000mL ddH 2 In O, autoclave at 121°C for 15 minutes, and store at 4°C.
[0050] (7) 50×TAE...
Embodiment 2
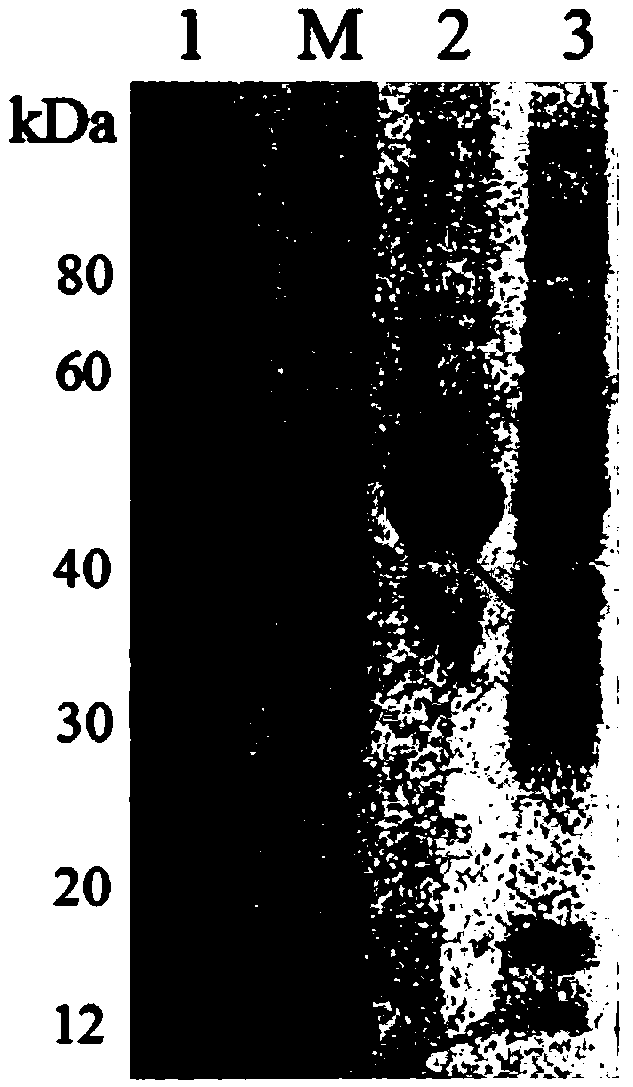
[0158] Example 2 (Induced expression and purification of Bifidobacterium longum adhesion protein)
[0159] Step 1: Induced expression of Bifidobacterium longum adhesion protein
[0160] Take the Bifidobacterium longum adhesion protein expression strain E.coli DH5αpGEX-4T-AIP2 prepared in Example 1 and use IPTG to induce expression, the specific steps are as follows:
[0161] (1) Pick a single colony of the strain that has been streaked on the plate, inoculate it into 3ml LB liquid medium, add Amp to the test tube to a final concentration of 100μg / ml, and culture it at 37°C with a constant temperature shaker at 180r / m for 8h .
[0162] (2) Transfer to 20ml low-salt LB liquid medium according to the inoculum size of 1%, add Amp to a final concentration of 100μg / ml, and cultivate at 37°C with a constant temperature shaker at 180r / m.
[0163] (3) When the cell density OD600nm=0.6-0.9, add the inducer IPTG to a final concentration of 1 mM, incubate at 37° C. with a constant tempe...
Embodiment 3
[0184] Embodiment 3 (a kind of aminoacid sequence is the protein preparation method of SEQ ID NO:1)
[0185] 1) Culture the recombinant Escherichia coli DH5α pGEX-4T-AIP2, add the inducer IPTG to a final concentration of 1.2mM when the OD600nm of the culture solution is 0.9, and then induce culture at 39°C for 5 hours under shaking conditions;
[0186] 2) collecting the bacteria, crushing, and collecting the supernatant;
[0187] 3) Take buffer A, equilibrate the glutathione agarose resin column with a flow rate of 3.5mL / min; take the supernatant from step 2), load the sample with a flow rate of 2mL / min; take buffer B, The flow rate of min is eluted, and the solution at the elution peak is collected as the eluent; the eluent is collected by ultrafiltration and concentrated, then passed through a desalting column, and then eluted with pure water at a flow rate of 3.5mL / min, and the eluent at the elution peak is collected. The solution is the second eluent, and then freeze-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com