Preparation method of low-temperature curing type waterborne amino acrylic resin
An aminoacrylate, water-based technology used in coatings to achieve low curing temperature and enhanced scratch resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
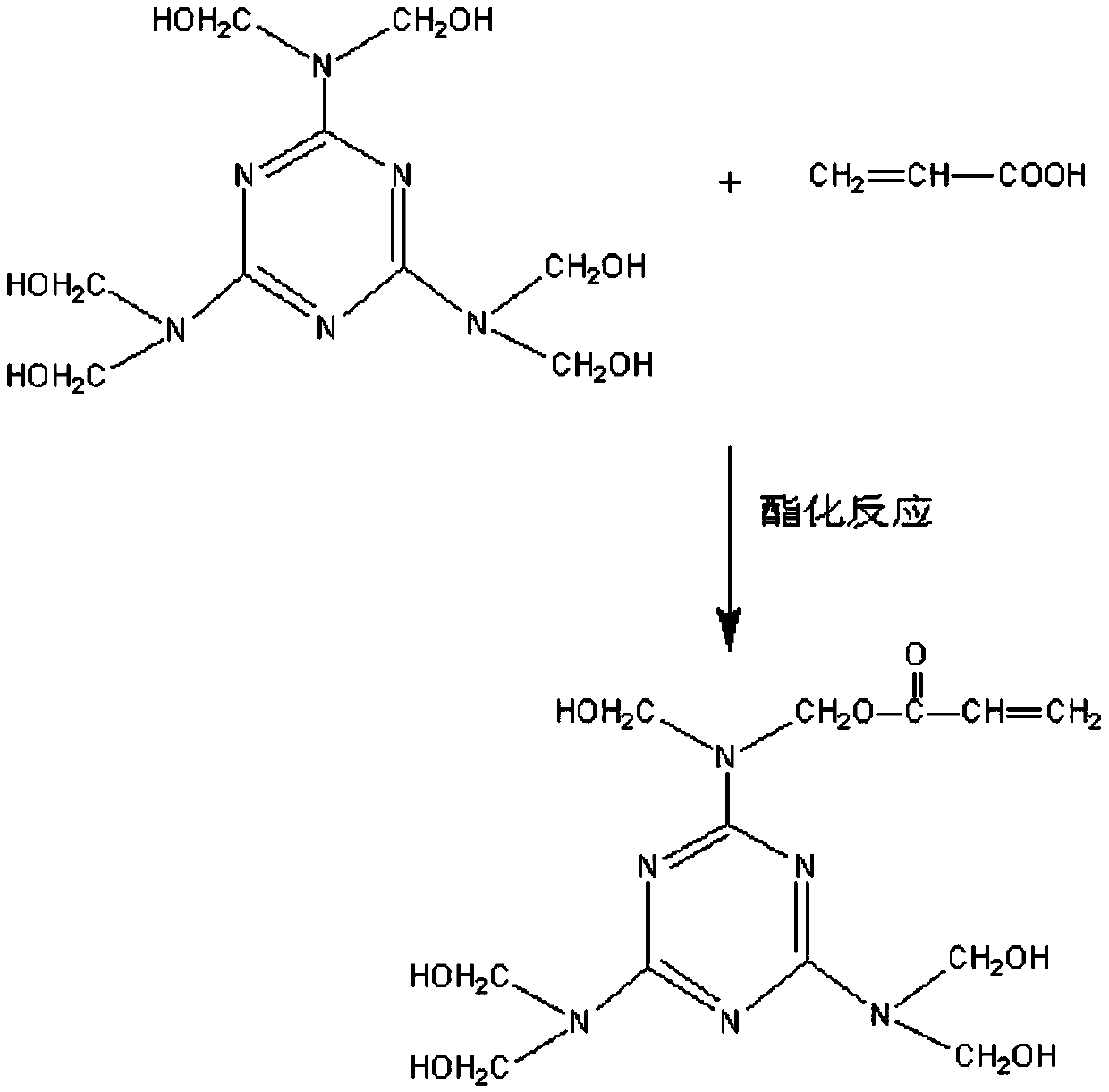
[0036] During the above esterification reaction, the molar ratio of acrylic acid and melamine formaldehyde resin was 1:1, the esterification reaction temperature was 110° C., and the reaction time was 1 h.
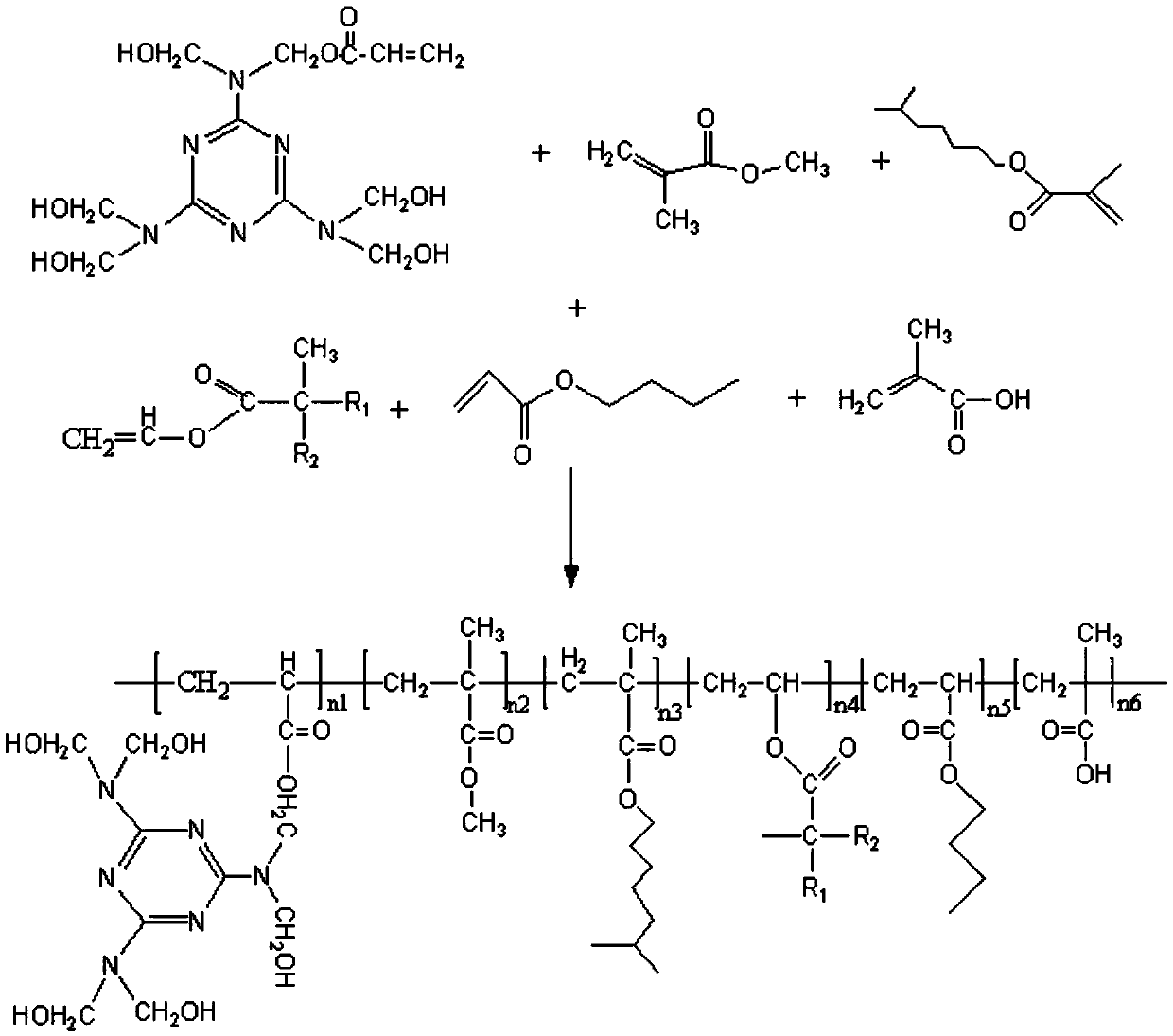
[0037] During the polymerization process, the amount of the aminoacrylic ester macromer is 4 parts; the amount of the methyl methacrylate is 5 parts; the amount of the isooctyl methacrylate is 30 parts; the tertiary carbonic acid The vinyl ester consumption is 30 parts; the described butyl acrylate consumption is 2 parts; the described α-methacrylic acid consumption is 3 parts; the described sodium bicarbonate consumption is 0.5 parts; the described ammonium persulfate consumption is 0.5 part; The described tert-butyl hydroperoxide consumption is 0.1 part; The described sodium bisulfoxylate formaldehyde consumption is 0.1 part; The emulsifier in the described emulsion polymerization is SR-10, and the consumption is 3 parts; The amount of deionized water used in the emulsio...
Embodiment 2
[0041] During the above esterification reaction, the molar ratio of acrylic acid and melamine formaldehyde resin was 1.2:1, the esterification reaction temperature was 130° C., and the reaction time was 3 hours.
[0042]During the polymerization process, the amount of the aminoacrylic ester macromer is 8 parts; the amount of the methyl methacrylate is 13 parts; the amount of the isooctyl methacrylate is 50 parts; the tertiary carbonic acid The consumption of vinyl ester is 50 parts; The consumption of described butyl acrylate is 5 parts; The consumption of described α-methacrylic acid is 5 parts; The consumption of described sodium bicarbonate is 0.7 part; The consumption of described ammonium persulfate is 0.7 part; The described tert-butyl hydroperoxide consumption is 0.3 part; The described sodium bisulfoxylate formaldehyde consumption is 0.3 part; The emulsifier in the described emulsion polymerization is SR-10, and the consumption is 5 parts; The amount of deionized water...
Embodiment 3
[0046] During the above esterification reaction, the molar ratio of acrylic acid and melamine formaldehyde resin was 1.12:1, the esterification reaction temperature was 115°C, and the reaction time was 1.3h.
[0047] During the polymerization process, the amount of the aminoacrylic ester macromonomer is 5 parts; the amount of the methyl methacrylate is 6 parts; the amount of the isooctyl methacrylate is 32 parts; the tertiary carbonic acid The consumption of vinyl ester is 32 parts; The consumption of described butyl acrylate is 2.5 parts; The consumption of described α-methacrylic acid is 3.3 parts; The consumption of described sodium bicarbonate is 0.53 parts; The consumption of described ammonium persulfate is 0.53 part; The described tert-butyl hydroperoxide consumption is 0.12 part; The described sodium bisulfite formaldehyde consumption is 0.12 part; The emulsifier in the described emulsion polymerization is SR-10, and the consumption is 3.3 parts; The amount of deionize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com