Monoclonal Antibodies and Their Applications
A monoclonal antibody, mouse anti-human technology, applied in the direction of antibodies, applications, anti-tumor drugs, etc., can solve the problems of immune infeasibility, achieve the effect of promoting proliferation, good curative effect and safety, and strengthening immune activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Embodiment 1: the preparation of hybridoma and carrier
[0145] 1. Antigen and Lymphocyte Preparation and Immunization
[0146] 1.1 Antigen preparation:
[0147] 1.1.1 Preparation of 293F cells expressing human PDL1 protein
[0148] Fusion of human PDL1 amino acid sequence and TEV-mlgG2aFc amino acid sequence, the amino acid sequence design is shown in SEQ ID No.13:
[0149] FTVTVPKDLYVVEYGSNMTIECKFPVEKQLDLAALIVYWEMEDKNIIQFVHGEEDLKVQHSSYRQRARLLKDQLSLGNAALQITDVKLQDAGVYRCMISYGGADYKRITVKVNAPYNKINQRILVVDPVTSEHELTCQAEGYPKAEVIWTSSDHQVLSGKTTTTNSKREEKLFNVTSTLRINTTTNEIFYCTFRRLDPEENHTAELVIPELPLAHPPNERKLENLYFQGPRGPTIKPCPPCKCPAPNLLGGPSVFIFPPKIKDVLMISLSPIVTCVVVDVSEDDPDVQISWFVNNVEVHTAQTQTHREDYNSTLRVVSALPIQHQDWMSGKEFKCKVNNKDLPAPIERTISKPKGSVRAPQVYVLPPPEEEMTKKQVTLTCMVTDFMPEDIYVEWTNNGKTELNYKNTEPVLDSDGSYFMYSKLRVEKKNWVERNSYSCSVVHEGLHNHHTTKSFSRTPGK
[0150] The amino acid sequence corresponding to the above-designed fusion protein (PDL1-TEV-mIgG2aFc) was artificially optimized for codons,...
Embodiment 2
[0184] Embodiment 2: the preparation of positive control antibody (PCAB)
[0185] 1. PCAb antibody sequence is as follows:
[0186] PCABH, as shown in SEQ ID No.14:
[0187] QVQLVQSGAEVKKPGASVKVSCKASGYTFTDYGFSWVRQAPGQGLEWMGWITAYNGNTNYAQKLQGRVTMTTDTSTSTVYMELRSLRSDDTAVYYCARDYFYGMDVWGQGTTVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPPCPSCPAPEFLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLGK
[0188] PCABL, as shown in SEQ ID No.15:
[0189] EIVLTQSPATLSSLSPGERATLSCRASQSVSSYLVWYQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPRTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESGNSQESVTEQDSKDSTYSLSSTLNTLSKADGYEKHKVYACEVTHS
[0190] 2. Artificially optimize the codons of the amino acid sequence corresponding to the above antibody se...
Embodiment 4
[0216] Example 4: Preparation and Identification of Monoclonal Antibody
[0217] 1. Preparation of antibodies by in vitro culture method
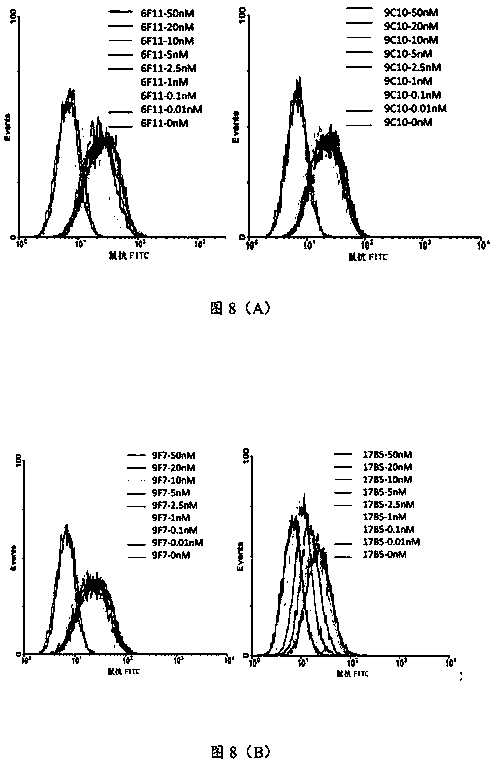
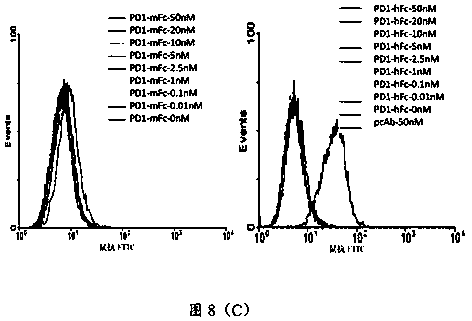
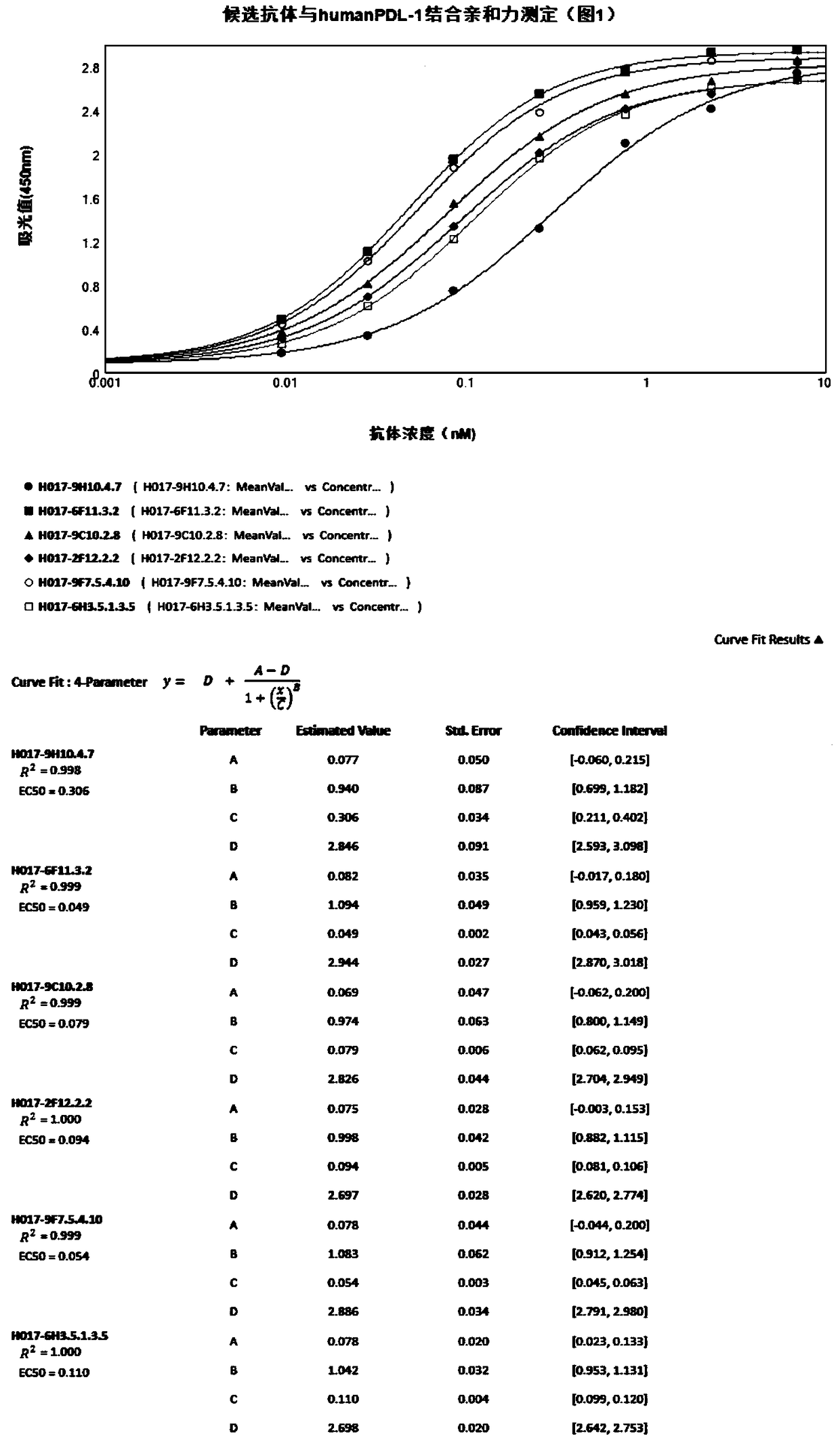
[0218] Resuscitate the DMEM medium containing 15% fetal bovine serum and 1% penicillin and streptomycin and use vials to culture H017-9H10.4.7, H017-6F11.3.2, H017-9C10.2.8, H017-2F12.2.2, H017-9F7 .5.4.10, H017-6H3.5.1.3.5, H017-13H10.2.6, H017-1B8.4.3.1, H018-17B5.5.1, after the cells are transferred to the medium bottle, replace with 10% fetal bovine serum, 1% Penicillin and streptomycin are cultured in DMEM medium, and the middle bottle is replenished to 20ml, and then the roller bottle is cultured to 200ml.
[0219] 2. Antibody Purification
[0220] Take out the cell supernatant cultured in the roller bottle for about 7 days, vacuum filter through a 0.22um mixed cellulose microporous membrane, store at 4°C, and perform protein A affinity chromatography for antibody purification. Loading: after equilibrating the purification column w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com