Divalproex sodium sustained-release agent composition and preparation method thereof
A technology of sodium divalproex and composition, which is applied in the directions of drug combination, anhydride/acid/halide active ingredient, pharmaceutical formulation, etc., can solve the problems of easy caking, difficult continuous production, easy sticking and flushing, etc. Long-lasting effect, reduced tablet size, and ease of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of divalproex sodium sustained-release tablet
[0050] Table 1 divalproex sodium sustained-release tablet (200 tablets) formula
[0051] Element
[0052] Preparation method: first weigh stearic acid, heat and melt, add divalproex sodium in the prescribed amount, stir evenly, add microcrystalline cellulose to granulate, cool to room temperature, pass through a 30-mesh sieve, and add lubricant magnesium stearate And silicon dioxide, tablet that is.
Embodiment 2
[0053] Embodiment 2: Preparation of divalproex sodium sustained-release tablet
[0054] Table 2 divalproex sodium sustained-release tablet (200 tablets) formula
[0055] Element
Dosage (200 tablets)
divalproex sodium
100.0g
hydrogenated castor oil
25.0g
20.0g
2.0g
silica
1.5g
[0056] Preparation method: first weigh 25.0g of hydrogenated castor oil, heat to 85°C, and continue to stir to melt, add the prescribed amount of divalproex sodium, mix well, add microcrystalline cellulose, stir and cool to room temperature, pass through 30 mesh Sieve, add lubricant magnesium stearate and silicon dioxide, and compress into tablets.
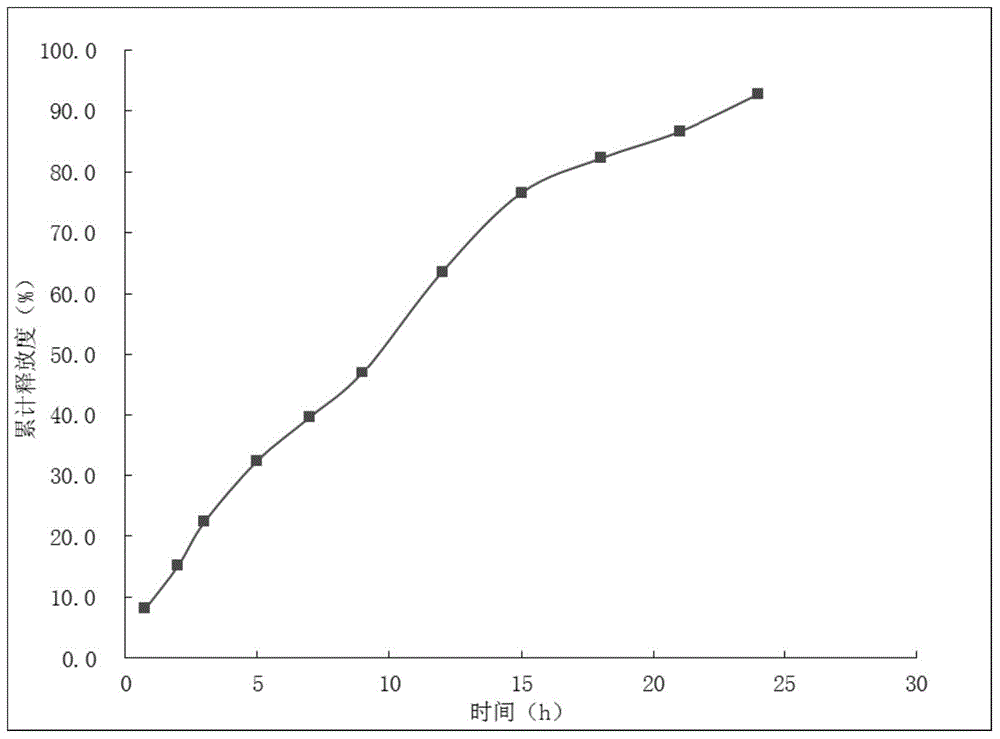
[0057] Under the conditions of USPII, 100rpm and 37°C, the drug release profile in the 0.1Mol / L hydrochloric acid of the sustained-release tablet can be found in figure 1 .
Embodiment 3
[0058] Embodiment 3: the preparation of divalproex sodium sustained-release tablet
[0059] Table 3 divalproex sodium sustained-release tablets (200 tablets) formula
[0060] Element
[0061] Preparation method: first weigh 25.0g of hydrogenated castor oil, heat to 85°C, and continue to stir to melt, add the prescription amount of divalproex sodium, a mixture of microcrystalline cellulose and lactose, stir evenly and cool to room temperature, pass 30 Mesh sieve, add lubricant magnesium stearate and silicon dioxide, and press into tablets to obtain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com