Spherical micro-nano ferric phosphate/carbon composite material and preparation method thereof
A carbon composite material, micro-nano technology, applied in the field of lithium-ion batteries, can solve the problems of ineffective material modification, uncontrollable mixing degree of reaction raw materials, immaturity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
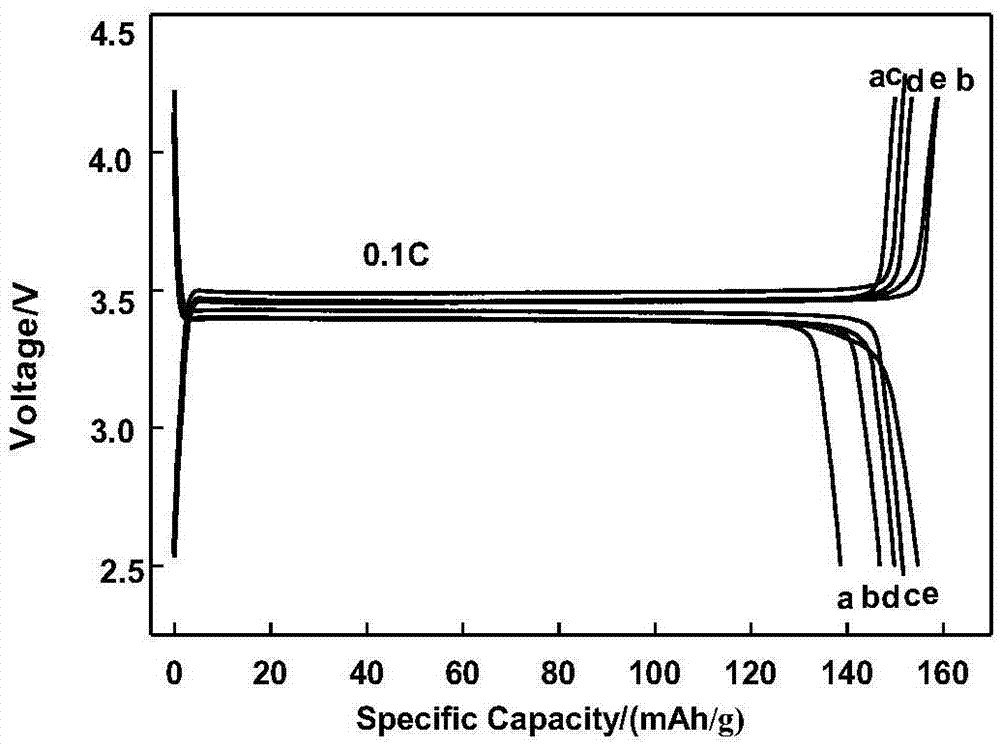
[0026] Weigh equimolar Fe(NO 3 ) 3 ·9H 2 O and H 3 PO 4 Prepare a 0.2mol / L mixed acid solution, measure a certain amount of ammonia and dilute to 0.5mol / L, and input the two into the crystallization reactor in parallel and continuously. The reaction temperature is 50°C, and the pH of the reaction system is adjusted and maintained at about 2 to control The stirring intensity is 200r / s, and the reaction solution overflows naturally after filling the reactor. After stirring for 10 hours, it is centrifuged, washed and dried to obtain FePO. 4 ·XH 2 O powder. The precursor powder is heat-treated at 500℃ for 10h to obtain nano-FePO 4 Precursor, using carbothermic reduction method to prepare nano-LiFePO 4 / C Cathode material. Weigh 12g FePO 4 Precursor, 2.982g Li 2 CO 3 Add 2.5g of sucrose and 5ml of deionized water to mix well, put it in a tube furnace, pass in high-purity nitrogen, heat treatment at 700℃ for 16h, and obtain nano-LiFePO after high-temperature carbothermic reduction rea...
Embodiment 2
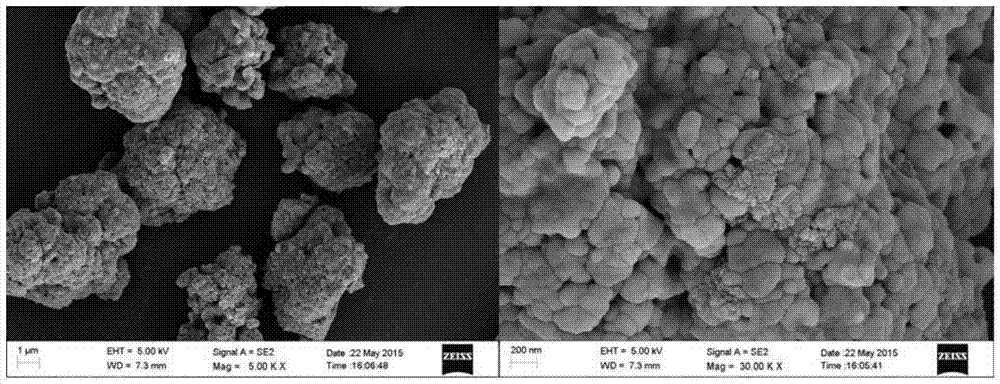
[0028] Weigh equimolar Fe(NO 3 ) 3 ·9H 2 O and H 3 PO 4 Make 0.2mol / L mixed acid solution, weigh FePO 4 After 0.5wt% of the theoretical mass of polyacrylamide is dissolved in the acid solution, a certain amount of ammonia is diluted to 0.5mol / L, and the two are continuously fed into the controlled crystallization reactor in parallel. The reaction temperature is 50°C, and the reaction system is adjusted and maintained. The pH is around 2, and the stirring intensity is controlled to 200r / s. The reaction solution overflows naturally after filling the reactor. After stirring for 10 hours, it is centrifuged, washed and dried to obtain 0.5wt% PAM-FePO 4 ·XH 2 O powder. PAM-FePO is obtained after heat treatment of the precursor powder at 500℃ for 10h 4 Precursor, prepare nano LiFePO modified by PAM according to the method of Example 1. 4 / C Cathode material. Such as figure 1 As shown, the PAM-FePO obtained in this example 4 The particle D50 is 4.3um, the carbon content is 4.4028%, and ...
Embodiment 3
[0030] Weigh equimolar Fe(NO 3 ) 3 ·9H 2 O and H 3 PO 4 Make 0.2mol / L mixed acid solution, weigh FePO 4 After the theoretical mass of 0.5wt% soluble starch is dissolved in the acid solution, measure a certain amount of ammonia to dilute to 0.5mol / L, and input the two in parallel and continuously into the controlled crystallization reactor. The reaction temperature is 50℃, and the pH of the reaction system is adjusted and maintained. At about 2, control the stirring intensity to 200r / s, and the reaction liquid will overflow naturally after filling the reactor. After stirring for 1 hour, after centrifugation, washing and drying, 0.5wt% soluble starch-FePO is obtained. 4 ·XH 2 O powder. The precursor powder is heat-treated at 600℃ for 10h to obtain spherical micro-nano FePO 4 Precursor, according to the method of Example 1 to prepare micro-nano LiFePO modified with soluble starch 4 / C Cathode material. Such as figure 1 As shown, the soluble starch-FePO obtained in this example 4 Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com