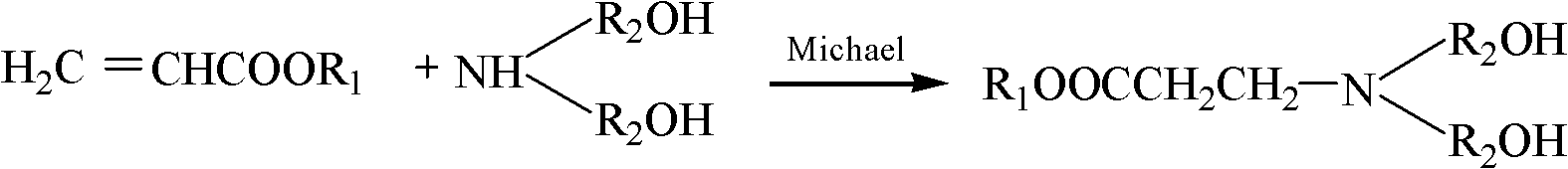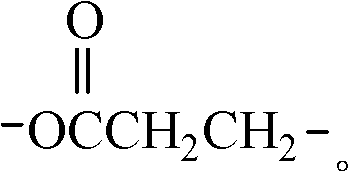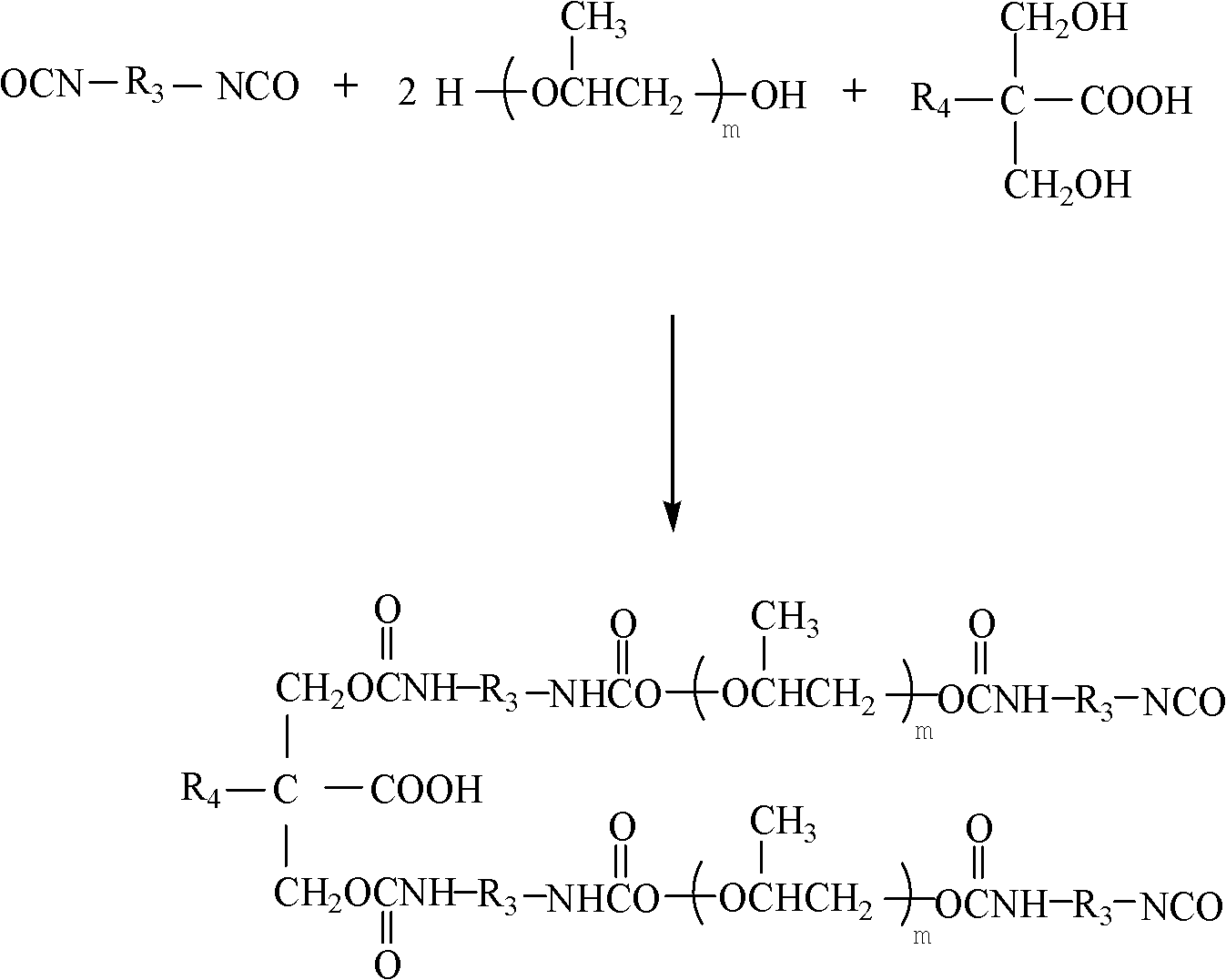Preparation method of hyperbranched water-based polyurethane hydroxy component
A polyurethane hydroxyl and water-based technology, applied in polyurea/polyurethane coatings, coatings, etc., can solve the problems of long drying time of water-based two-component polyurethane paint film, low molecular weight of the final product, poor hardness, etc., and achieve high branching degree , Increase the cross-linking density, increase the effect of contact probability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh 86.09g methyl acrylate, 105.14g diethanolamine and 100ml methanol in a four-necked flask, the mixture is at room temperature and logical N 2 Under normal circumstances, after stirring for 30 minutes, the temperature was raised to 35°C and maintained for 4 hours, and then vacuumed to remove methanol to obtain a colorless and transparent oily N,N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer.
[0042] Weigh 1.36g of pentaerythritol, 7.6492g of N, N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer and 0.04g of p-toluenesulfonic acid in a four-necked flask, heat the mixture to 120h°C for 2h, then add 15.2984g of N , N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer and 0.08g p-toluenesulfonic acid were placed in a four-necked flask, kept at 120h°C for 2h, and finally vacuumed to remove the generated methanol to obtain a light yellow oil The second generation of hydroxyl-terminated hyperbranched poly(amine-ester).
[0043]Weigh 1.48g of ...
Embodiment 2
[0046] Weigh 86.09g methyl acrylate, 105.14g diethanolamine and 100ml methanol in a four-necked flask, the mixture is at room temperature and logical N 2 Under normal circumstances, after stirring for 30 minutes, the temperature was raised to 35°C and maintained for 4 hours, and then vacuumed to remove methanol to obtain a colorless and transparent oily N,N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer.
[0047] Weigh 1.36g pentaerythritol, 7.6492g N, N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer and 0.04g p-toluenesulfonic acid in a four-neck flask, heat the mixture to 120h°C for 1h, then add 15.2984gN , N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer and 0.08g p-toluenesulfonic acid in a four-necked flask, kept at 120h°C for 1h, and continued to add 30.5968g N, N-dihydroxyethyl-3 - methylalanine monomer and 0.16g p-toluenesulfonic acid in a four-necked flask, kept at 120h°C for 2h, and finally evacuated to remove the generated methanol t...
Embodiment 3
[0051] Weigh 86.09g methyl acrylate, 105.14g diethanolamine and 100ml methanol in a four-necked flask, the mixture is at room temperature and logical N 2 Under normal circumstances, after stirring for 30 minutes, the temperature was raised to 35°C and maintained for 4 hours, and then vacuumed to remove methanol to obtain a colorless and transparent oily N,N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer.
[0052] Weigh 1.36g pentaerythritol, 7.6492g N, N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer and 0.04g p-toluenesulfonic acid in a four-neck flask, heat the mixture to 120°C for 0.5h, then add 15.2984 gN, N-dihydroxyethyl-3-aminopropionic acid methyl ester monomer and 0.08g p-toluenesulfonic acid in a four-necked flask, kept at 120°C for 0.5h, and continued to add 30.5968g N, N-dihydroxyethyl -Methyl 3-aminopropionate monomer and 0.16g p-toluenesulfonic acid were placed in a four-necked flask, kept at 120°C for 1h, and then 61.1936g N, N-dihydroxyeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com