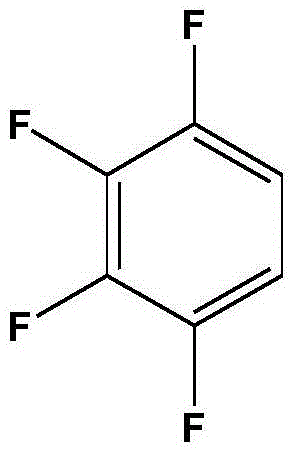
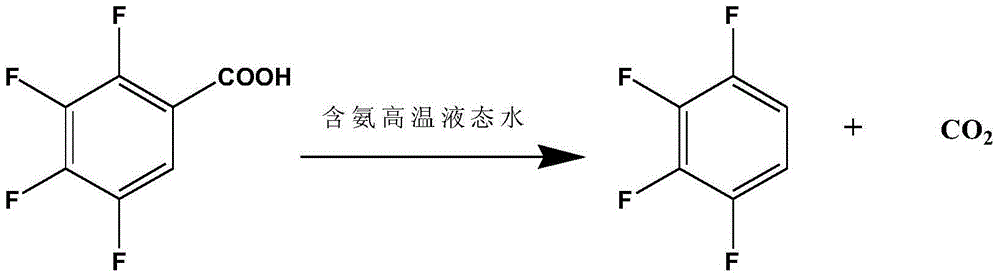
Preparation method of 1,2,3,4-tetrafluorobenzene from 2,3,4,5-tetrafluorobenzoic acid
A technology of tetrafluorobenzoic acid and tetrafluorophthalic acid, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc. Alkaline, promoting ionization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 300g of deionized water and 37.5g of 2,3,4,5-tetrafluorobenzoic acid into a 500mL intermittent autoclave, start stirring, and heat up to 240°C for 4 hours of decarboxylation reaction; after the decarboxylation reaction is completed, open the exhaust valve to relieve pressure , cooled to room temperature, after standing, the liquid-liquid layer was separated to obtain an organic phase and an aqueous phase, and the organic phase was rectified to obtain 14.5 g of 1,2,3,4-tetrafluorobenzene, and the purity of the product was 98.1% through HPLC analysis. The yield was 50.0%.
Embodiment 2
[0027] Add 300g of ammonia solution with an ammonia concentration of 0.5g / L and 42.8g of 2,3,4,5-tetrafluorobenzoic acid into a 500mL batch-type high-pressure reactor, start stirring, and heat up to 210°C for 5 hours of decarboxylation reaction; after the decarboxylation reaction is completed , open the exhaust valve to relieve the pressure, and recover the ammonia in the kettle; cool down to room temperature, and after standing still, the liquid-liquid layer is separated to obtain an organic phase and an aqueous phase, and the organic phase is rectified to obtain 1,2,3,4-four 25.3 g of fluorobenzene, the purity of the product analyzed by HPLC was 98.5%, and the yield was 76.4%.
Embodiment 3
[0029] Add 300g of ammonia solution with an ammonia concentration of 1g / L and 50.0g of 2,3,4,5-tetrafluorobenzoic acid into a 500mL intermittent high-pressure reactor, start stirring, and heat up to 220°C for 4.5 hours of decarboxylation reaction; after decarboxylation is completed, Open the exhaust valve to relieve the pressure, recover the ammonia in the kettle; cool down to room temperature, and after standing still, the liquid-liquid layer is separated to obtain an organic phase and an aqueous phase, and the organic phase is rectified to obtain 1,2,3,4-tetrafluoro 32.5 g of benzene, the purity of the product analyzed by HPLC was 98.5%, and the yield was 84.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com