Preparation and application of coliphage MS2 internal standard quality control product and kit
A technology of Escherichia coli and bacteriophage, which is applied in the field of pathogen diagnosis, can solve the problems of easy contamination of the laboratory by plasmids, cumbersome operation, unsuitable monitoring, etc., and achieves the effects of easy in vitro culture, high safety, and convenient transportation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of coliphage MS2 internal standard quality control product
[0046] 1. Preparation of host bacteria
[0047] Use an inoculation loop to pick out a little Escherichia coli ATCC15597 strain and inoculate it on #271 solid medium for overnight culture; pick 1 loop of bacteria into 10mL #271 liquid medium, and cultivate overnight to obtain a culture solution containing host bacteria;
[0048] 2. Preparation of monoclonal phage MS2
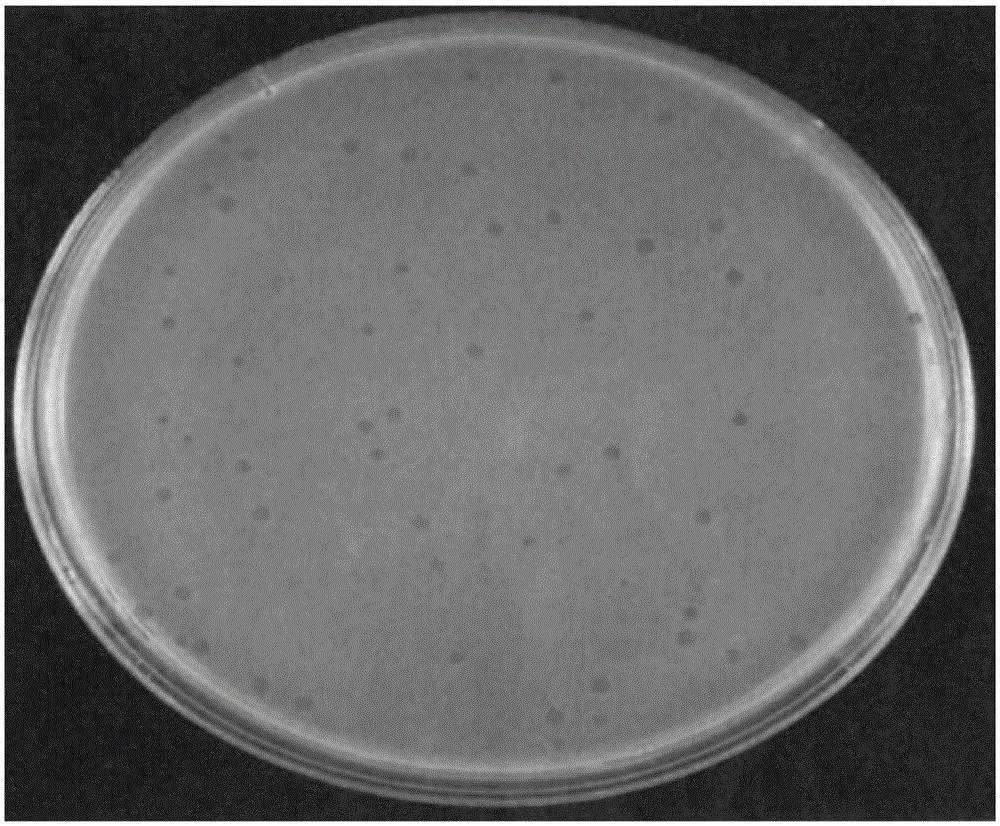
[0049] Take out 0.5ml of phage, and use #271 liquid medium for 10-fold serial dilution, take 100uL of the serially diluted phage liquid and mix with 300uL of the culture medium containing the host bacteria prepared in step 1, let it stand for 15 minutes, and mix with 4mL of 45℃ Mix the #271 semi-solid medium, then pour it into the prepared #271 solid medium, and put it into a 37°C incubator for 18-24 hours after solidification. Pick a single phage plaque into the liquid medium containing Escherichia coli cultured by th...
Embodiment 2
[0075] Embodiment 2: Homogeneity test of coliphage MS2 internal standard quality control product
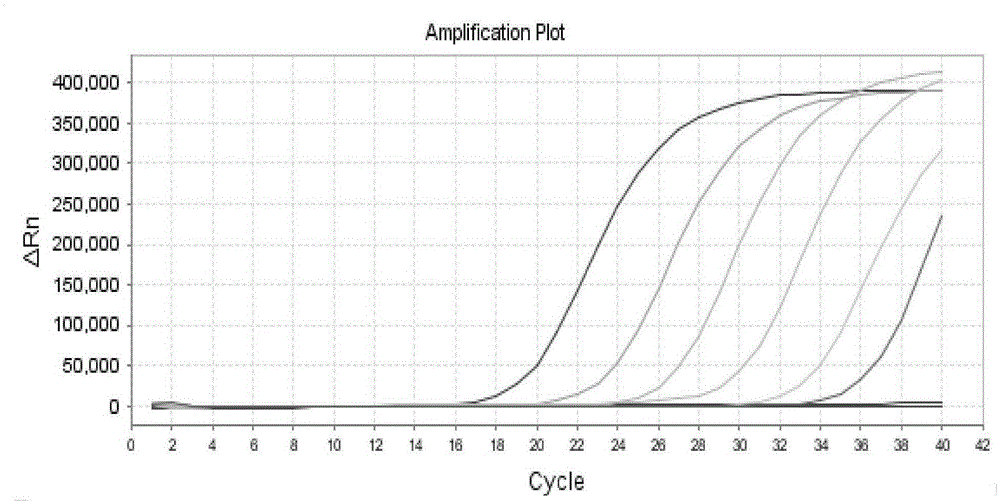
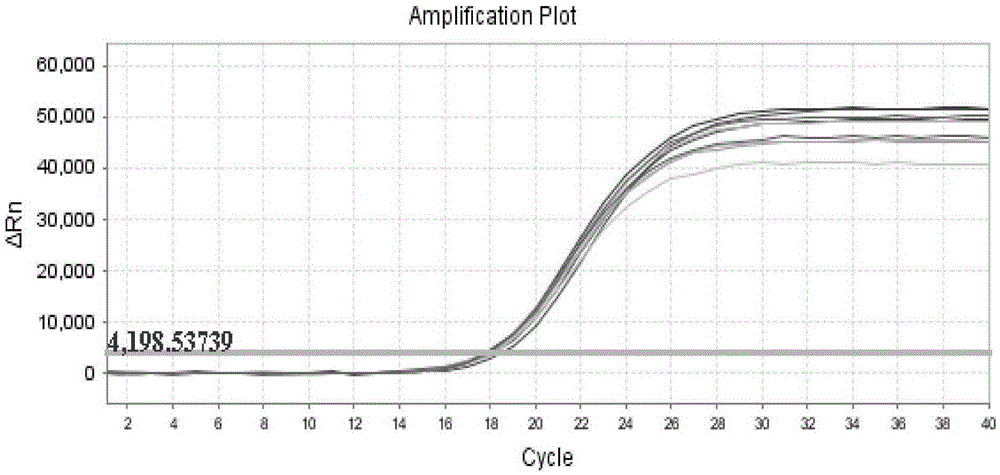
[0076] Take 9 tubes of standard bacteriophage MS2 prepared in Example 1 stored at -20°C and dilute with 200uL sample diluent, shake, mix and centrifuge, take 50uL each for fluorescent RT-PCR detection, the detection system and method are the same as in Example 1, and carry out Statistical analysis, to evaluate the uniformity of phage MS2 internal standard quality control, the experimental results are shown in the appendix figure 2 , and the data are organized in the following table:
[0077] freeze-dried product
1
2
3
4
5
6
7
8
9
Ct value
17.94
18.19
18.02
18.04
17.99
18.0
18.28
18.64
18.01
[0078] The relative standard deviation, that is, the coefficient of variation, is used to evaluate the uniformity, which is represented by CV:
[0079] Average value X=(Ct1+Ct2+Ct3+····+Ct9) / 9 ...
Embodiment 3
[0085] Example 3: Stability analysis of coliphage MS2 internal standard quality control product
[0086] Take 3 tubes of phage MS2 internal standard quality control prepared in Example 1 stored at -20°C at different time points for fluorescence RT-PCR determination, the detection system and method are the same as in Example 1, and perform statistical analysis of phage MS2 at each sampling time point The stability of the internal standard quality control product; samples were taken every two months in the first 8 months, and samples were taken every other month in the next 8 months, and the fluorescent RT-PCR assay was carried out for statistical analysis. The results are shown in the table below:
[0087]
[0088] The regression analysis of variance results of the RT-PCR detection Ct value of coliphage MS2 are shown in the following table:
[0089]
[0090] The F value is less than the F critical value, indicating that the change of the phage MS2 internal standard quali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com