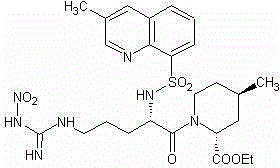
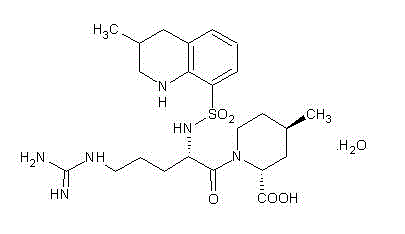
Preparation method of argatroban intermediate
A compound, -quinolinesulfonyl technology, applied in the direction of peptides, etc., can solve problems such as large-scale production of difficult argatroban
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 42.4g (0.10mol) of compound IV, 13.5g (0.10mol) of 1-hydroxybenzotriazole, 26mL (0.15mol) of diisopropylethylamine, 550mL of dichloromethane and 35mL of DMF in a 1000ml three-necked flask , stirred and dissolved, cooled to 0°C-5°C with ice water, added 18.8g (0.11mol) of compound V and O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid ( TBTU) 32.1g (0.10mol), stirred and reacted at 0°C to 10°C for 30 minutes. Transfer to a separatory funnel, add 240 ml of water, shake and let stand to separate layers, take the organic phase, add 200 mL of dichloromethane to the water phase for extraction, combine the organic phases, dry with anhydrous magnesium sulfate, filter, and concentrate under reduced pressure , add 150 ml of diethyl ether, stir and crystallize at 0°C~-5°C for 1h. 54.9 g of the off-white solid of the target object was obtained by filtration, the yield was 95.0%, and the content was 97.6% (HPLC normalization method).
Embodiment 2
[0022] Add 21.2g (0.05mol) of compound IV, 6.8g (0.05mol) of 1-hydroxybenzotriazole, 17.5mL (0.10mol) of diisopropylethylamine, and 320mL of tetrahydrofuran into a 500ml three-necked flask, and stir to dissolve , cooled to 0°C to 5°C with ice water, added 10.3g (0.06mol) of compound V and 19.0g (HBTU) of benzotriazole-N,N,N',N'-tetramethylurea hexafluorophosphate (HBTU) ( 0.05mol), stirred and reacted at 0°C to 10°C for 60 minutes. Transfer to a separatory funnel, add 160 ml of saturated saline, shake and let stand to separate layers, take the upper organic phase, dry it with anhydrous magnesium sulfate, filter, concentrate under reduced pressure, add 80 ml of diethyl ether, and store at 0 ° C ~ -5 After stirring and crystallizing at ℃ for 40 minutes, 24.9 g of the off-white solid of the target object was obtained by filtration, with a yield of 86.1% and a content of 97.3% (HPLC normalization method).
Embodiment 3
[0024] Add 21.2g (0.05mol) of compound IV, 6.8g (0.05mol) of 1-hydroxybenzotriazole, 14.0mL (0.10mol) of triethylamine, 300mL of acetonitrile into a 500ml three-necked flask, stir to dissolve, and dissolve in ice water Cool to 0°C-5°C, add 10.3g (0.06mol) of compound V and 26.0g (0.05mol) of benzotriazol-1-yl-oxytripyrrolidinylphosphine hexafluorophosphate (PyBOP), Stir the reaction at 10°C for 60 minutes, concentrate under reduced pressure, add 200 mL of dichloromethane and transfer it to a separatory funnel, then add 160 mL of saturated saline, shake and leave to separate layers, take the lower layer of dichloromethane and wash with anhydrous sulfuric acid Dry magnesium, filter, concentrate under reduced pressure at 35 ° C, add 80 ml of ether, stir and crystallize at 0 ° C ~ -5 ° C for 40 minutes, filter to obtain 25.6 g of off-white solid of the target object, the yield is 88.6%, and the content is 97.5%. (HPLC normalization method).
[0025] With PyBOP as condensing agent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com