Milrinone pharmaceutical composition and preparation method thereof
A technology of composition and pesticide, applied in the field of milrinone pharmaceutical composition and its preparation, can solve problems affecting product quality, increasing production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] prescription composition
[0033]
[0034] Preparation Process
[0035] ⑴ Concentrated
[0036] First weigh 1 g of milrinone, add 2.56 g of L-lactic acid (calculated as dry weight of lactic acid), and stir at 70°C for 5 minutes to fully dissolve milrinone in L-lactic acid to form milrinone lactic acid.
[0037] Then add an appropriate amount of water for injection at 70°C, add 9.0g of sodium chloride, and after stirring thoroughly, add water for injection to 800ml.
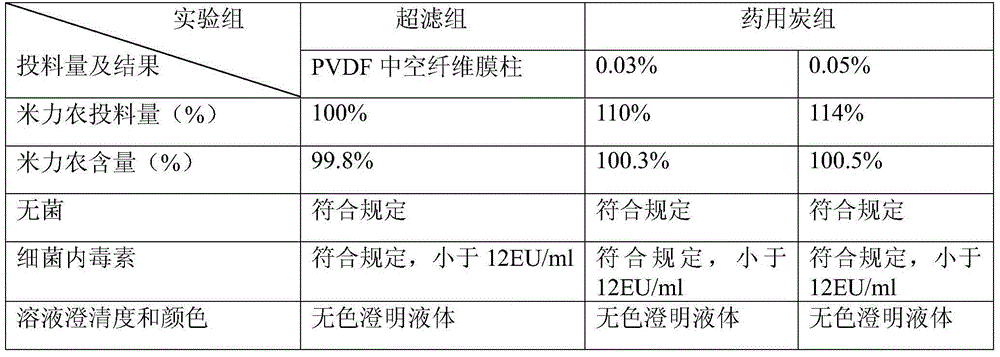
[0038] ⑵ Depyrogenation
[0039] A hollow fiber membrane column made of polyvinylidene fluoride with a molecular weight cut-off range of 6000 Daltons is used for ultrafiltration.
[0040] ⑶ rare match
[0041] Add water for injection to 1000ml, and use L-lactic acid solution to adjust the pH value of the liquid to within the range of 2.8 to 3.4. Check the intermediate, and fine filter through a 0.2μm filter membrane after passing the test.
[0042] ⑷ Filling
[0043] The fine filtrate is filled in...
Embodiment 2
[0047] prescription composition
[0048]
[0049] Preparation Process
[0050] ⑴ Concentrated
[0051]A Weigh 1 g of milrinone, add 2.9 g of L-malic acid, and stir at 70°C for 5 min to fully dissolve milrinone in L-lactic acid to form milrinone malate.
[0052] B Add an appropriate amount of water for injection at 70°C, add 9.0g of sodium chloride, and stir thoroughly, then add water for injection to 800ml.
[0053] ⑵ Depyrogenation
[0054] A hollow fiber membrane column made of polyvinylidene fluoride with a molecular weight cut-off range of 6000 Daltons is used for ultrafiltration.
[0055] ⑶ rare match
[0056] Add water for injection to 1000ml, and use L-lactic acid solution to adjust the pH value of the liquid to within the range of 2.8 to 3.4. Check the intermediate, and fine filter through a 0.2μm filter membrane after passing the test.
[0057] ⑷ Filling
[0058] The fine filtrate is filled into ampoules with a specification of 5ml or 10ml.
[0059] ⑸ Steri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com