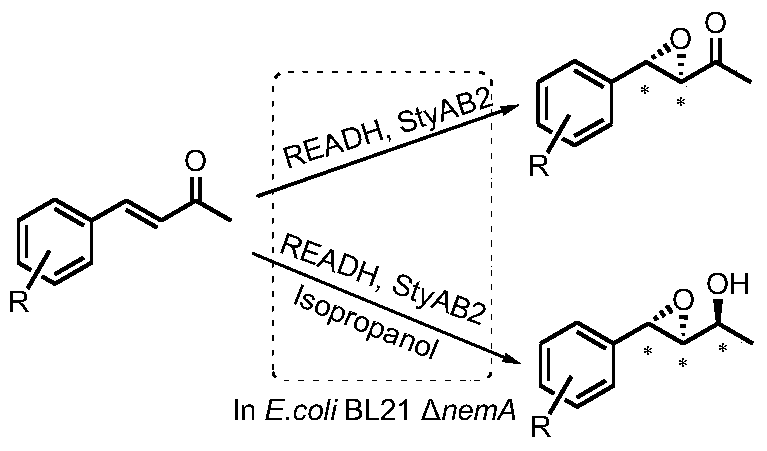
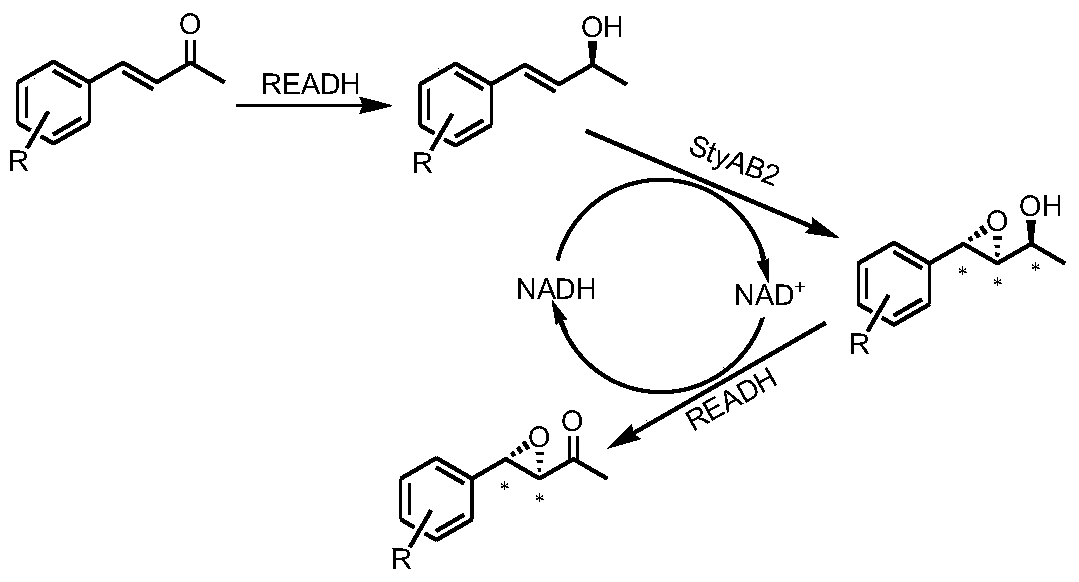
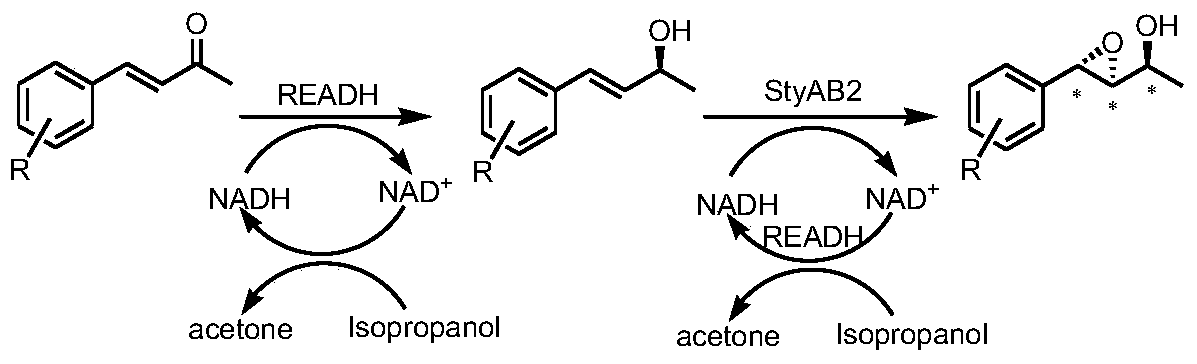
A tunable multienzyme cascade for the synthesis of optically pure allylic epoxy ketones or alcohols
An epoxy ketone and optical technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, and the use of vectors to introduce foreign genetic materials, etc., to achieve high ee and de values, efficient biosynthesis routes, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Construction of biocatalyst E.coli BL21ΔnemA (pRSFD-REAB)
[0028] (1) Construction method of Escherichia coli expression strain E.coli BL21ΔnemA
[0029] Since the N-acetylmaleimide reductase expressed by the nemA gene in the E.coli BL21 genome can efficiently reduce the C=C bond of the substrate ɑ,β-unsaturated ketone, we introduced λRed recombination and knocked out E.coli The nemA gene in the BL21 genome.
[0030] Using P1 / P3 and P4 / P2 as primers (Table 1) and E. coli BL21 genomic DNA as a template, homologous sequences on both sides of the nemA gene were amplified respectively. PCR amplification conditions: pre-denaturation at 94°C for 5min, denaturation at 94°C for 30s, annealing at 55°C for 30s, extension at 72°C for 0.5min, 30 cycles, and final extension at 72°C for 10min.
[0031] Using FRTf and FRTr as primers (Table 1) and plasmid pKD4 as a template, the kanamycin resistance gene fragment was amplified. Due to the different lengths of the fragme...
Embodiment 2
[0043] Example 2: Synthesis of (3R,4S)-4-phenyl-3,4-epoxy-2-butanone
[0044] Take 1.5 g of the bacteria from Example 1 and resuspend in 10 ml potassium dihydrogen phosphate / dipotassium hydrogen phosphate buffer (0.1M, pH=7.0), add 20 mM model substrate 4-phenyl-3-butene-2 - Ketone (1a in Table 2), 30°C, 230rpm, react for 2h. Take 1ml of the reaction solution, add 800μl of ethyl acetate to extract, centrifuge at 12,000 for 2min, take the organic phase, add appropriate amount of anhydrous sodium sulfate to dry, distill under reduced pressure, dissolve the product in 1ml of isopropanol, and filter by organic membrane for HPLC detection. Shimadzu Prominence LC-20AD system Daicel chiral column AD-H was used, and the detector was PDA. Detection conditions, isopropanol:n-hexane=10:90, 0.5ml / min, column temperature 35°C. The product is (3R,4S)-4-phenyl-3,4-epoxy-2-butanone, the conversion rate is >99%, and the enantioselectivity ee value is >99%. The product was determined by NMR-...
Embodiment 3
[0046] Embodiment 3: Synthesis of other chiral allyl epoxy ketones
[0047] Take 2 g of the bacteria from Example 1 and resuspend in 10 ml potassium dihydrogen phosphate / dipotassium hydrogen phosphate buffer (0.1 M, pH=7.0), add 10 mM substrate, react at 30° C., 230 rpm for 2 h. The reaction solution treatment method is the same as in Example 2.
[0048] Substrates include 4-phenyl-3-buten-2-one and derivatives obtained by halogen and methyl substitution at o- / m- / p-position respectively (Table 2). Conversion 70-99% and ee 85-99%. It has good conversion efficiency and excellent enantioselectivity ee value (>99%) for most substrates.
[0049]
[0050] Table 2
[0051]
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com