A kind of conjugated polyacetylene containing perylene imide and preparation method thereof
A technology of conjugated polyacetylene and peryleneimide, which is applied in the field of preparation of conjugated polyacetylene, can solve the problems of limiting the comprehensive performance of photoelectric devices, poor solution film processing performance, and low utilization rate of sunlight, etc., and achieve excellent spectrum Absorption and energy level regulation performance, good environmental processing performance, and the effect of simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of Poly(1), the synthetic route is as follows:
[0064]
[0065] In this example, the reaction tube was always kept under a nitrogen atmosphere, and the solvents used were all dried and purified.
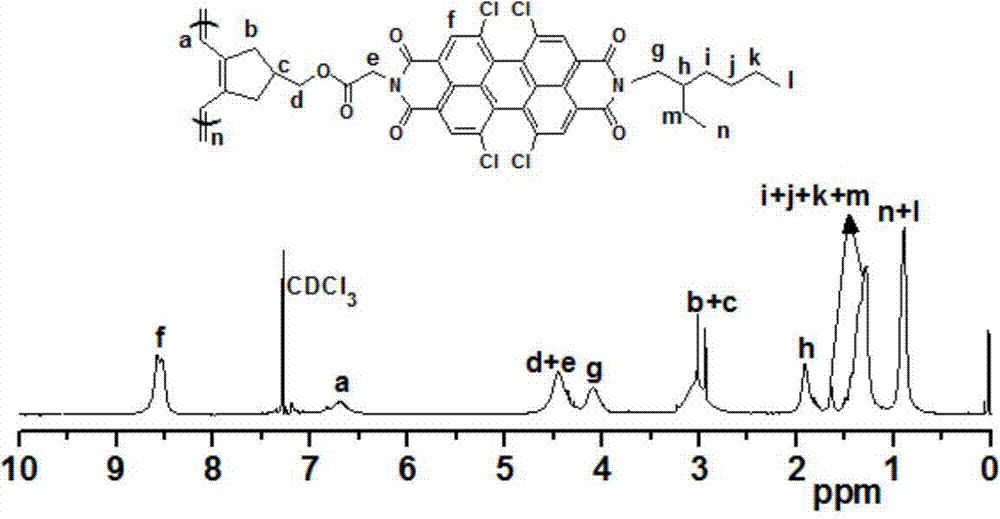
[0066] Add 1,6,7,12-tetrachloro-3,4:9,10-perylenetetracarboxylic dianhydride (2.65g, 5mmol) and 30mL toluene into a 100mL reaction flask, stir well, then add glycine (0.41g , 5.4mmol) and 2-ethylhexylamine (0.69g, 5.4mmol), reflux for 48h. Cool down to room temperature, add 150mL dichloromethane, wash 3 times with water (50mL×3), and wash the organic phase with MgSO 4After drying and removing the solvent, the crude product was separated by column chromatography using dichloromethane / ethyl acetate = 10:1 as the eluent to obtain 1.62 g of dark red solid 1a with a yield of 25%.
[0067] Under a nitrogen atmosphere, add the above product 1a (2.34g, 3.4mmol), 4-hydroxy-1,6-heptadyne (0.83g, 6.8mmol) and 100mL dichloromethane into a 100mL reaction flask, stir to diss...
Embodiment 2
[0070] The polymerization conditions are the same as in Example 1, only the solvent is changed to tetrahydrofuran. 154 mg of black solid poly(1) was obtained with a yield of 96%, and the others were the same as in Example 1.
Embodiment 3
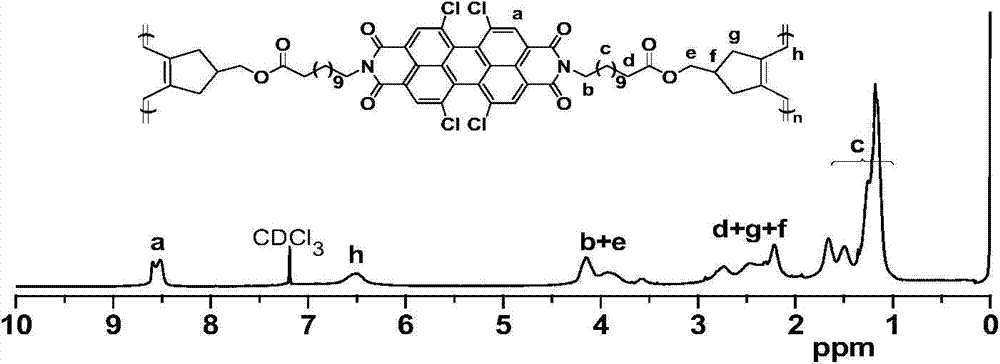
[0072] The synthesis of Po1y(2), the synthetic route is as follows:
[0073]
[0074] Under nitrogen atmosphere, in a 150mL reaction flask, add 1,6,7,12-tetrachloro-3,4:9,10-perylenetetracarboxylic dianhydride (3.01g, 5.6mmol), glycine (3.00g, 40mmol) and 40mL propionic acid, stir until the sample is evenly dispersed, and stop the reaction after 40h of reflux reaction. Cool to room temperature, filter, wash the filter cake with water until the filtrate is neutral, put it in a vacuum drying oven at 80°C and dry to constant weight to obtain 3.20 g of red powder 2a with a yield of 91%. The product is directly put into the next step without further purification dehydration reaction.
[0075] Under a nitrogen atmosphere, in a 100mL reaction flask, add 2a (2.20g, 3.5mmol), 4-hydroxymethyl-1,6-heptadiyne (1.65g, 13.5mmol), 40mLN,N-dimethylformamide and 10 mL of chloroform. Under the condition of ice bath, EDCI.HCl (1.56g, 8.4mmol) and DMAP (0.13g, 1.1mmol) were added, the tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com