Oxygen carrier for chemical cycle dry gas reforming and its preparation method and application
A dry gas reforming and oxygen carrier technology, which is applied in chemical instruments and methods, inorganic chemistry, bulk chemical production, etc., can solve the problems of low cycle reactivity, reduced reactivity, high reaction temperature, etc., and achieve low cost, The effect of promoting the formation and improving the catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
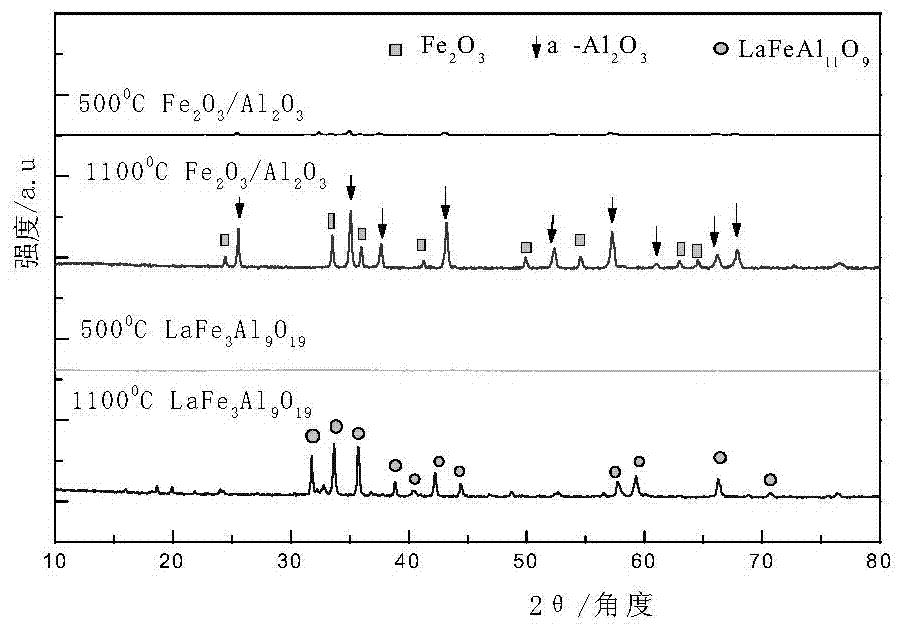
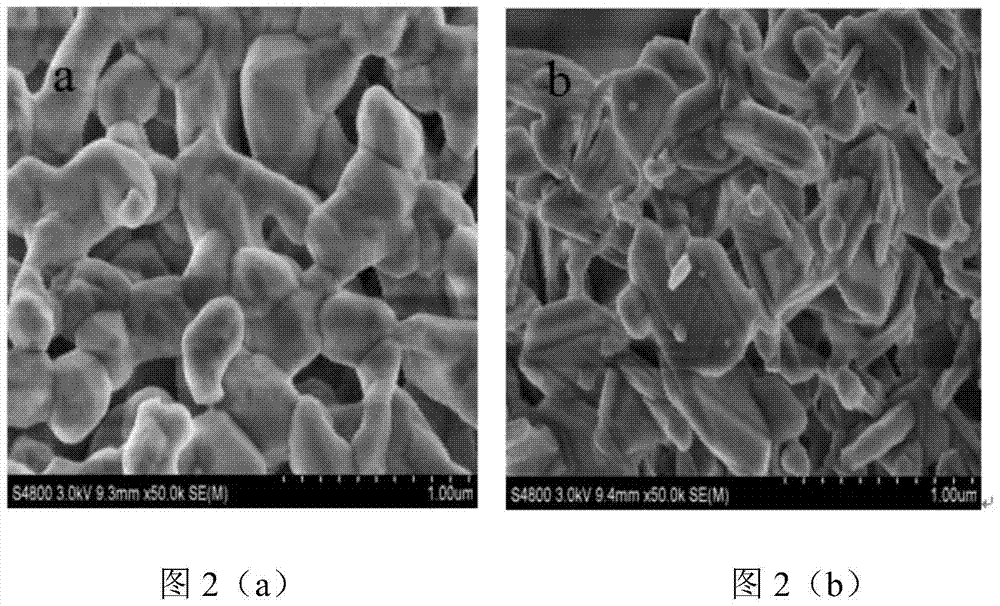
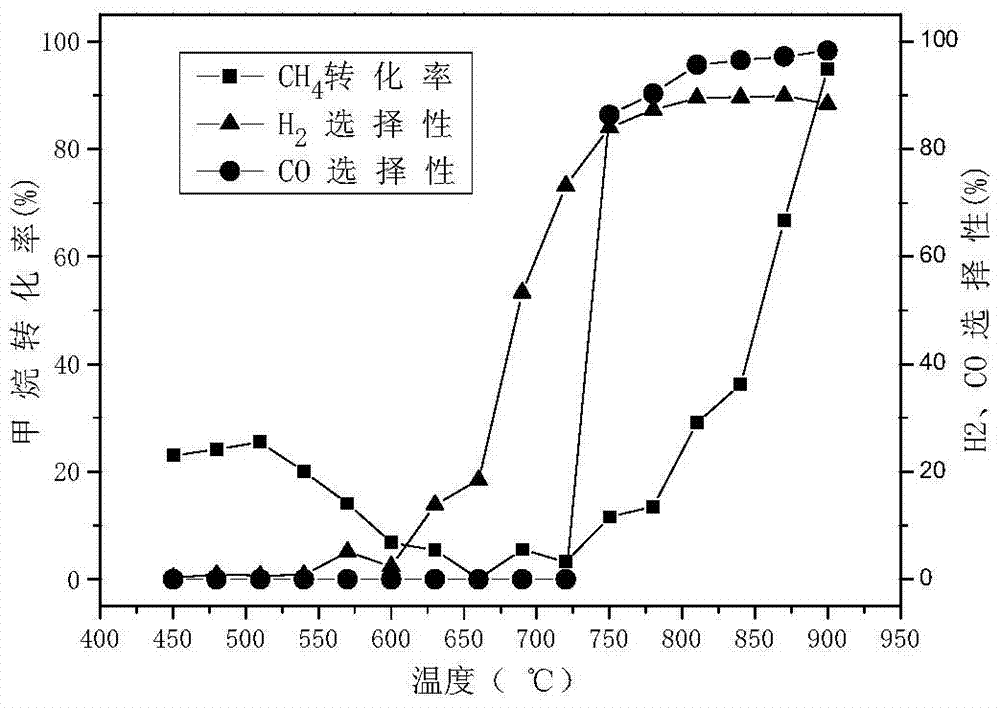
[0043] Preparation of hexaaluminate (LaFe 3 Al 9 o 19 ) oxygen carrier. Proceed as follows:
[0044] 2.54 grams of lanthanum nitrate (La(NO 3 ) 2 ), 7.1 grams of iron nitrate (Fe(NO 3 ) 3 9H 2 O) and 19.78 grams of aluminum nitrate (Al(NO 3 ) 3 9H 2 O) were respectively dissolved in deionized water at 60° C. to form lanthanum nitrate solution, iron nitrate solution, and aluminum nitrate solution each at 1 mol / L. After mixing the lanthanum nitrate solution and the ferric nitrate solution evenly, use 0.1mol / L nitric acid to adjust the pH value to 1, then add the aluminum nitrate solution, after mixing evenly, a mixed solution is obtained, and quickly add the mixed solution to excess saturated ammonium carbonate In the solution, stir rapidly (stirring speed is 250r / min) at 60°C for 6 hours, filter to obtain precipitate, dry the precipitate at 120°C for 12 hours, then roast the precipitate, and then cool down to room temperature naturally. Wherein, the amount of ammoni...
Embodiment 2
[0049] Preparation of Ba 1-x La x FeAl 11 o 19 (x=0-1, step size is 0.2) hexaaluminate oxygen carrier, labeled B 1-x L x F (LF when x=1, BF when x=0), the Ba(NO 3 ) 2 , La(NO 3 ) 3 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O and Al(NO 3 ) 3 9H 2 O was dissolved in deionized water at 60°C to form 1 mol / L Ba(NO 3 ) 2 solution, La(NO 3 ) 3 solution, Fe(NO 3 ) 3 solution, Al(NO 3 ) 3 solution. Ba(NO 3 ) 2 solution, La(NO 3 ) 3 solution, Fe(NO 3 ) 3 After the solution is mixed evenly, use 2mol / L nitric acid to adjust the pH value to 1, then add aluminum nitrate solution, after mixing evenly, a mixed solution is obtained, and the mixed solution is quickly added to an excess of 1mol / L saturated ammonium carbonate solution. After stirring vigorously at 60°C for 6 hours, filter the precipitate, wash the precipitate with deionized water for 3-5 times and dry at 120°C for 12 hours; grind, then transfer to a muffle furnace and bake at 500°C for 4 hours , and then baked at 12...
Embodiment 3
[0055] Preparation of LaFe x Al 12-x o 19 (x = 0-4, step size 1) hexaaluminate oxygen carrier, labeled LF x (When x=0, LFxA=LA). The molar ratio is 1:x:(12-x) La(NO 3 ) 3 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O and Al(NO 3 ) 3 9H 2 O was respectively dissolved in deionized water at 60°C to form a 1mol / L solution. Mix lanthanum nitrate solution and ferric nitrate solution evenly, use 1mol / L nitric acid to adjust PH=1, then add aluminum nitrate solution, after mixing evenly, quickly add to excess saturated ammonium carbonate solution, at 60°C 400r / Stir vigorously for 6 hours; filter and separate, wash with deionized water for 3-5 times, and then dry at 120°C for 12 hours; mill, transfer to a muffle furnace and bake at 500°C for 4 hours, then heat up to 1100°C for 4 hours, Let it cool down to room temperature naturally. Wherein, the amount of ammonium carbonate in the saturated ammonium carbonate solution is 1.6 times of the required molar amount of ammonium carbonate when lan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com