Poly-carbonyl curcumin derivative containing carboxyl group, preparation method and application thereof
A technology of carbonyl curcumin and derivatives, applied in the field of medicine, can solve the problems of low bioavailability, low solubility, large dosage, etc., achieve the effect of inhibiting β-secretase, protecting nerve cells, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
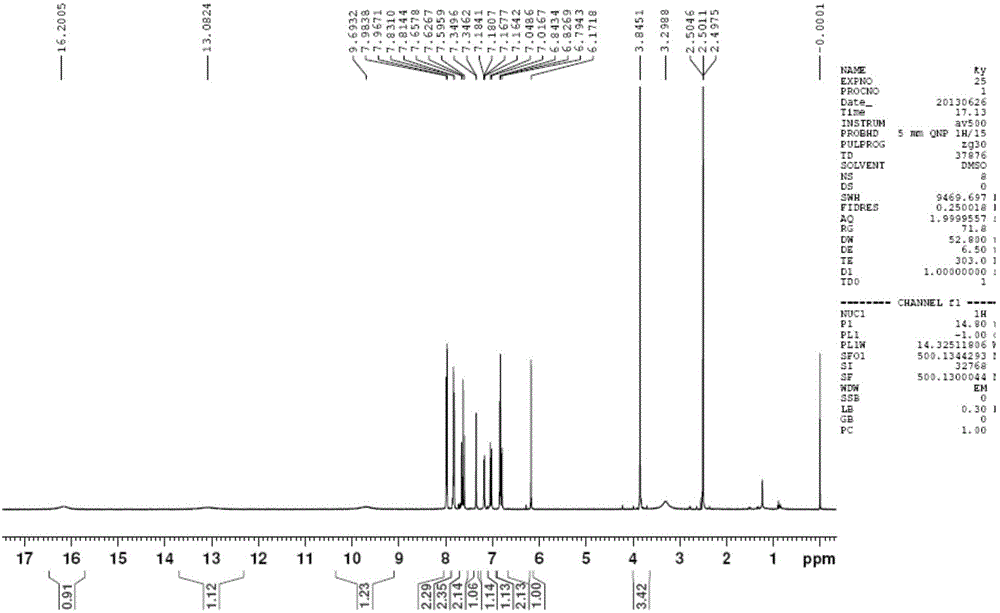
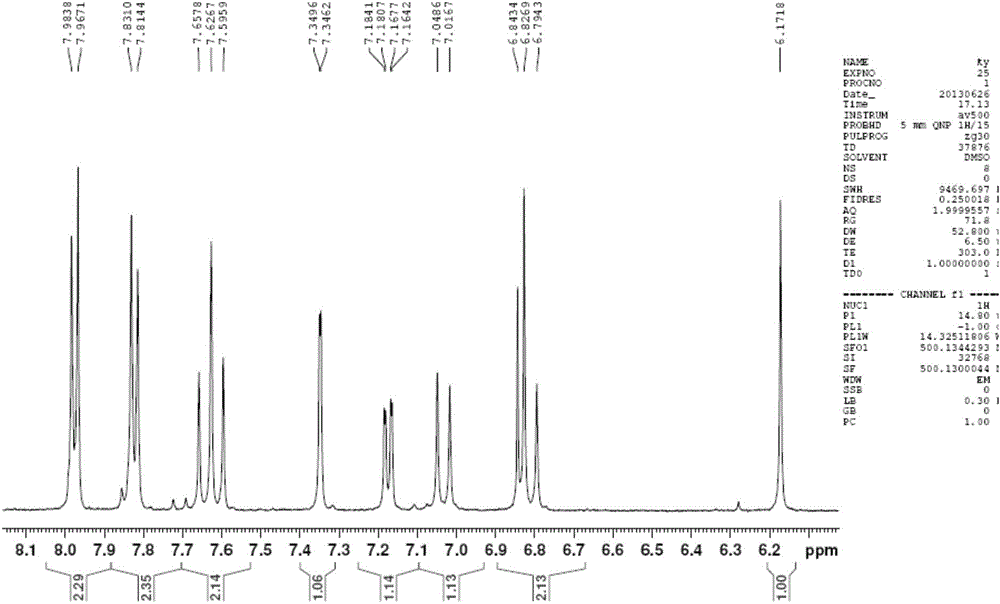
[0038] (1) Dissolve acetylacetone (1g, 10mmol) and boron oxide (0.49g, 7mmol) in 10mL of ethyl acetate, stir at 40°C for 30min, then add vanillin (1.522g, 10mmol), p-formyl Methyl benzoate (1.642g, 10mmol) and tributyl borate (4.6g, 20mmol) were stirred for 30min. n-Butylamine (1mL) was dissolved in 10mL of ethyl acetate and added dropwise slowly for 30min. After the dropwise addition was completed, it was stirred at 40°C for 4h, and left overnight to make the reaction complete. The mixture was hydrolyzed with aqueous hydrochloric acid (0.4N, 15mL) at 60°C for 1 h, the aqueous phase was extracted three times with ethyl acetate, the organic phases were combined, washed with water until neutral, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and flash silica gel Column chromatography (petroleum ether: acetone = 2:1) gave an orange-yellow solid product, 532 mg, with a yield of 14%. That is the intermediate (Ⅱ).
[0039] (2) Dissolve i...
Embodiment 2
[0045] (1) Dissolve acetylacetone (1g, 10mmol) and boron oxide (1g,) in 10mL of ethyl acetate, stir at 40°C for 30min, then add vanillin (1.522g, 10mmol), p-formylbenzoic acid Methyl ester (1.642g, 10mmol) and tributyl borate (4.6g, 20mmol), continued stirring for 30min. n-Butylamine (1mL) was dissolved in 10mL of ethyl acetate and added dropwise slowly for 30min. After the dropwise addition was completed, it was stirred at 40°C for 4h, and left overnight to make the reaction complete. The mixture was hydrolyzed with aqueous hydrochloric acid (0.4N, 15mL) at 60°C for 1 h, the aqueous phase was extracted three times with ethyl acetate, the organic phases were combined, washed with water until neutral, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and flash silica gel Column chromatography (petroleum ether: acetone = 2:1) gave an orange-yellow solid product, which was intermediate (Ⅱ).
[0046] (2) Dissolve intermediate (II) (174mg, ...
Embodiment 3
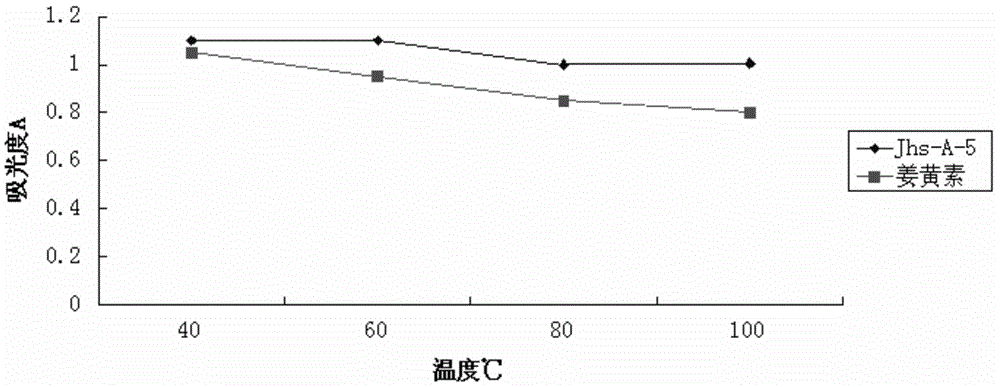
[0047] Embodiment 3 The drug efficacy test of carboxy polycarbonyl curcumin derivatives of the present invention
[0048] Experiment 1: Activity screening of acetylcholinesterase inhibitors
[0049] 1. The purpose of the experiment: to accurately screen samples with acetylcholinesterase inhibitor activity.
[0050] 2. Experimental materials
[0051] 2.1 Drugs and experimental reagent consumables
[0052] Sample: carboxyl-containing polycarbonyl curcumin derivative (abbreviation: Jhs-A-5), prepared according to the method in Example 1.
[0053] Curcumin reference substance, Nanjing Zelang Pharmaceutical Technology Co., Ltd.
[0054] Acetylcholinesterase (Electric eel), sigma
[0055] Thioacetylcholine iodide, Sigma
[0056] 5,5-dithiobisnitrobenzoic acid (DTNB), sigma
[0057] Tacrine hydrochloride (Tacrine hydrochloride), sigma company
[0058] Disodium hydrogen phosphate, Shantou Xilong Chemical Factory Co., Ltd.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com