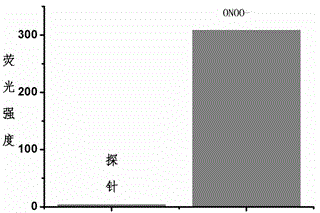
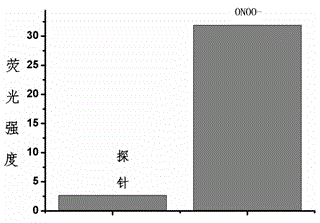
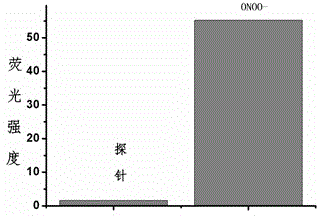
Fluorescent probe for detecting peroxynitrite, and preparation and application of fluorescent probe
A nitroso peroxide, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as poor specificity, inability to apply in vivo detection, etc., and achieve good stability and good biofilm. The effect of permeability and detection of high signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of fluorescent probe molecule Ia-1
[0042] 1g of o-aminothiophenol (8 mmol) and o-hydroxybenzaldehyde (8 mmol) were stirred in 8 mL of methanol for two hours at room temperature, and filtered by suction to obtain a white solid 2-(2,3-dihydrobenzo[d ]thiazol-2-yl)phenol. That is, 1a-1 was obtained with a yield of 50%. 1 H NMR (500 MHz, DMSO) δ9.85 (s,1H), 7.4 (d,1H), 7.1(t,1H), 6.95(d,1H), 6.75-6.85(m,3H), 6.65(d ,2H), 6.55(t,1H), 6.45(s,1H).
[0043] .
Embodiment 2
[0044] Embodiment 2: the preparation of fluorescent probe molecule Ib-1
[0045]o-Aminothiophenol (0.5g, 3.6mmol) and o-Hydroxybenzaldehyde (0.438g, 3.5mmol) were dissolved in 5mL of methanol solution, 37% hydrogen peroxide (15.75mmol) was added dropwise under ice-cooling and then Then add 37% hydrochloric acid (9.2 mmol) dropwise, react at room temperature for two hours and filter with suction, recrystallize from ethanol to obtain intermediate 2-(benzo[d]thiazol-2-yl)phenol II-1, yield 70 %; Intermediate II-1 (0.5g, 2 mmol) was first dissolved by stirring with 20 ml of anhydrous dichloromethane, replaced with nitrogen, and first added dropwise pyridine solution (328 μL, 4 mmol) to the reaction solution under ice bath, Then trifluoromethanesulfonic anhydride (0.863 g, 3 mmol) was slowly added dropwise to the reaction solution at a uniform speed, and stirred at room temperature for 3-4 hours. After the reaction was completed, it was extracted with ethyl acetate, dried over anh...
Embodiment 3
[0047] Embodiment 3: the preparation of fluorescent probe molecule Ib-2
[0048] Intermediate 2-(Benzo[d]thiazol-2-yl)phenyl triflate (0.25 g, 0.7 mmol) and 4-((tert-butyldimethylsilyl)oxy) -3-Methoxyaniline (0.25 g, 0.8 mmol), palladium acetate (3% mmol), BINAP (4.5% mmol) and cesium carbonate (1.4 mmol) were dissolved in 4 mL of toluene and reacted at 80 °C for 16 Hours, suction filtration, column chromatography purification. The key intermediate of yellow 2-(benzo[d]thiazol-2-yl)-N-phenylaniline was obtained with a yield of 80%; the intermediate was N-methylated and dehydroxylated to obtain the probe molecule 1b -2, yield 65%. 1 H NMR (500 MHz, DMSO) δ10.4 (s, 1H), 8.15 (d, 1H), 8.05 (d, 1H), 7.8 (d, 1H), 7.55 (t, 1H), 7.45 (t, 1H ), 7.3(m, 2H), 7.0 (3, 1H), 6.85 (3, 1H), 6.7(d, 1H), 6.5 (dd, 1H) ,3.85 (s, 3H), 3.8 (s, 3H) .
[0049] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com