Lentiviral vector with efficient infectivity and multiplication capacity promoting effect for T cells and hematopoietic stem cells
A carrier and recombinant virus technology, applied in the field of medical biology, can solve the problems of complex experimental procedures and low infection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1: Construction of recombinant lentiviral vector
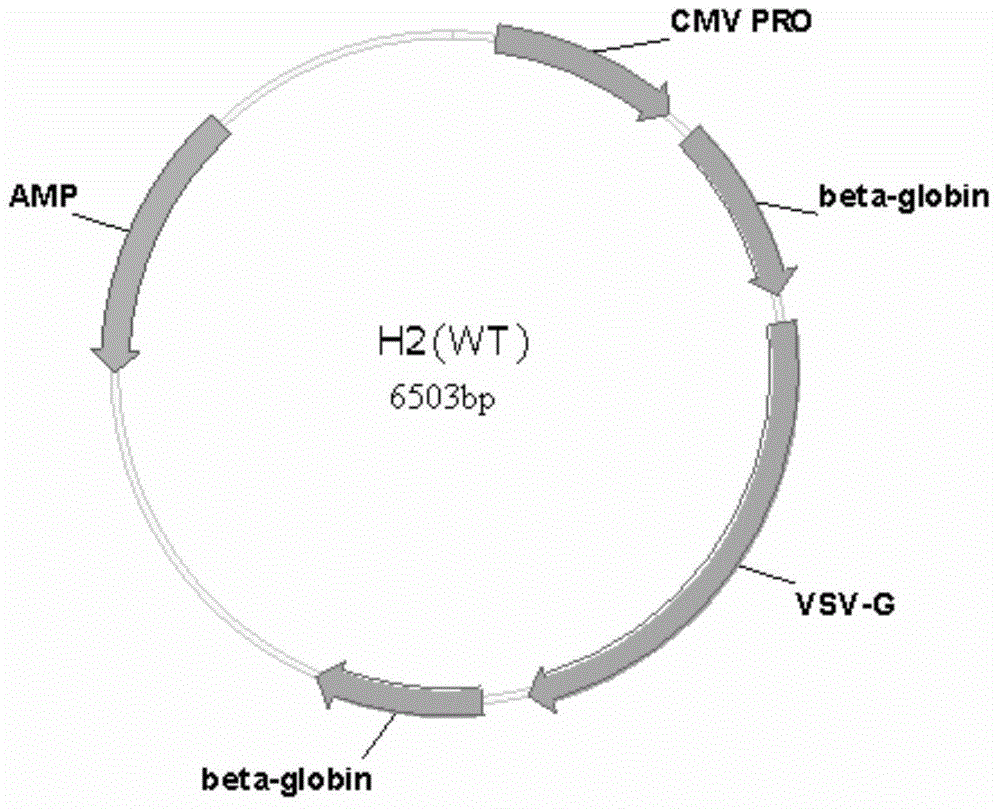
[0156] The construction of the recombinant lentiviral vector is based on the viral packaging helper plasmid H2 (purchased from Addgene, namely pVSV-G) carrying the VSVG gene.
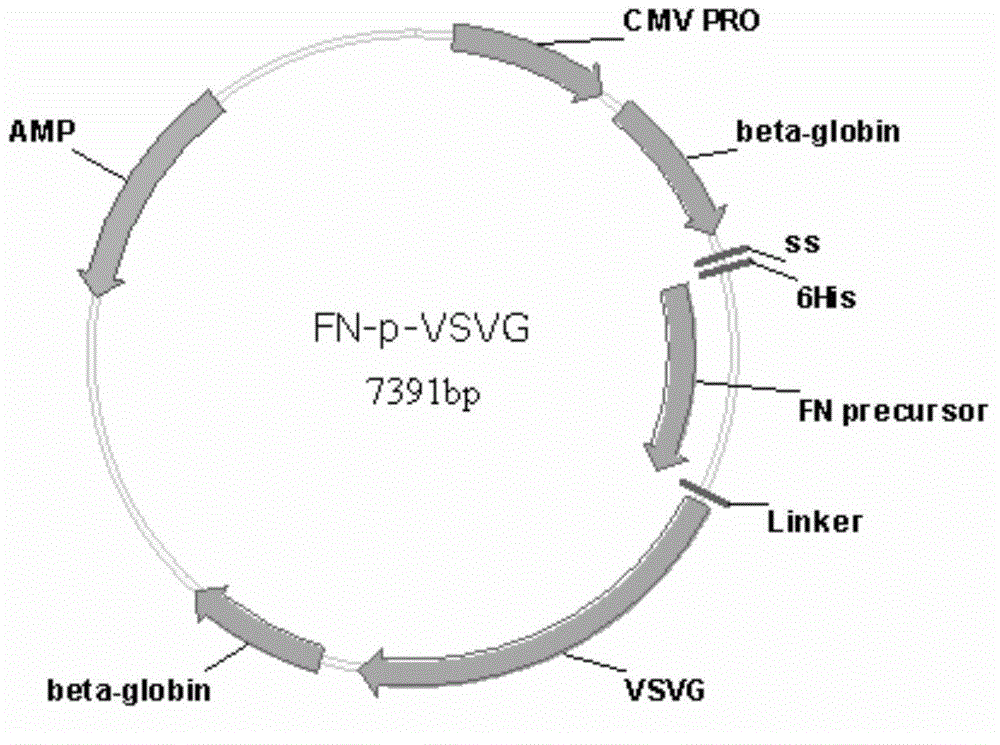
[0157] Construction of FN-p-VSVG recombinant lentiviral vector:
[0158] a) obtain the FN-p gene fragment:
[0159] Whole gene synthesis of signal peptide-6His-FN-p-flexible peptide fragment (the signal peptide nucleotide sequence information is SEQ ID NO.: 8, the 6His nucleotide sequence information is SEQ ID NO.: 9, FN-p nucleoside The acid sequence information is SEQ ID NO.: 3, the flexible peptide nucleotide sequence information is SEQ ID NO.: 10), using the whole gene synthesis plasmid as a template, design the FN-p gene primer, forward primer 1 (5'TTCCTCGACGGATCC CTCGAG ATGAAGTGCCTTTTGTACTTAGCCTTTTTATTCATTGG3', SEQ ID NO.:11) and reverse primer 2 (5'TGGTGAACTTTGAACCGCCTCCGCTCGAGCCAC3', SEQ ID NO.:12), PCR amplification, PCR con...
Embodiment 2
[0204] Example 2: Packaging of recombinant lentiviruses
[0205] 1. Supply HEK-293T cells (purchased from ATCC) according to the 24h passage cycle, change the medium before transfection, and use an electric pipette to replace 5ml of DMEM medium containing 2% FBS for each plate of cells.
[0206] 2. To prepare a transfection system, add plasmid H1 (purchased from Addgene, 12 μg / dish, the same below for a 10 cm cell culture dish), plasmid H2 (the total amount of H2 plasmid is 10 μg / dish, in this example, Recombinant and wild-type H2 plasmids in different ratios), vector plasmid (lentiviral vector containing exogenous genes, purchased from Addgene, 24 μg / plate), CaCl 2 (50μl / plate), and finally add 2×HBS (500μl / plate) dropwise while shaking on a vortex shaker, and the transfection system is 1ml / plate. The recombinant H2 is a 1:1 mixture of FN-p-VSVG recombinant plasmid and CS1-VSVG recombinant plasmid.
[0207] 3. Pipette the transfection system carefully, mix well, take 1000 μ...
Embodiment 3
[0211] Example 3: Recombinant lentivirus infection of T lymphocytes
[0212] Infection experiments were performed according to conventional methods known to those skilled in the art. A brief description of the infection steps is as follows:
[0213] 1. Peripheral blood mononuclear lymphocytes (PBMC) were obtained through the blood apheresis system > 1x10 7 cells.
[0214] 2. Use complete medium (TexMACS medium + 5% autologous serum) to adjust part of the cell suspension so that the final concentration is 0.7 × 10 6 / ml, and add interleukin 2 (IL-2), penicillin (penicillin) and streptomycin (streptomycin), OKT3 and bovine serum albumin (BSA), so that the final concentration is 600IU / ml, 100U / ml, 100μg / ml and 0.2%.
[0215] 3. Gently mix the resuspended cells, take out 0.4ml of cell suspension (total cell volume 0.28×10 6 ) into a 1.5mL centrifuge tube.
[0216] 4. Add the required virus, and calculate the amount of virus added according to the MOI of 10.
[0217] 5. Gen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com