Fluorescence-enhanced probe compound preparation method and trivalent chromium ion detection method
A technology of fluorescent probes and compounds, applied in the field of fluorescent probes, can solve the problems of complex design and synthesis of fluorescent probes, low fluorescence quantum yield, low detection limit, etc., and achieve high stability and selectivity of probe products. and high sensitivity, simple pre-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
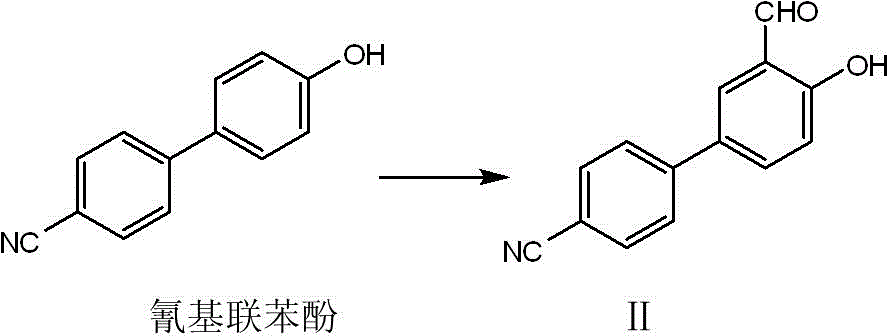
[0033] Embodiment 1, Synthetic experiment of intermediate product II
[0034] Take 0.31 g of cyanobiphenol and 0.22 g of anhydrous magnesium chloride and put them in a flask under nitrogen protection, add 0.61 g of anhydrous triethylamine and 20 ml of acetonitrile, stir to make them evenly mixed, add 0.66 g of paraformaldehyde, and heat to reflux 6 hours. After the reaction was stopped, a small amount of water was added to quench it, acidified with excess dilute hydrochloric acid, the mixture was extracted 3 times with ethyl acetate, dried, and purified by silica gel column to obtain product II. H NMR spectrum: 1 H NMR (400 MHz, CDCl 3 ) δ ppm : 11.11 (s, 1H), 9.99 (s, 1H), 7.78 (m, 4H), 7.66 (dt, 2H, J=8.55, 1.99), 7.12 (d, 1H, J=8.46).
Embodiment 2
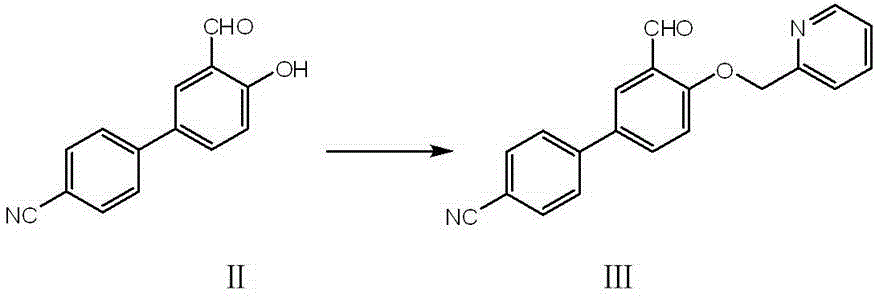
[0035] Embodiment 2, Synthesis experiment of intermediate product Ⅲ
[0036] Take 1.1 grams of sample II, 0.82 grams of 2-chloromethylpyridine hydrochloride, 2.5 grams of anhydrous potassium carbonate, and 0.36 grams of potassium iodide, put them together in a flask, and add 35 milliliters of acetonitrile to dissolve them. After heating to reflux for 6 hours, the reaction was stopped, and the inorganic salt was removed by filtration. The obtained crude product was concentrated and purified on a silica gel column to obtain product III. H NMR spectrum: 1 H NMR (400 MHz, CDCl 3 ) δ ppm : 10.65 (s, 1H), 8.64 (d, 1H, J=4.42), 8.12 (d, 1H, J=2.53), 7.78 (m, 2H), 7.73 (d, 2H, J=8.58) , 7.67 (dd, 2H, J=8.66, 2.25), 7.55 (d, 1H, J=8.06), 7.29 (dd, 1H, J=7.62, 5.28), 7.18 (d,1H, J=8.74), 5.39 (s, 2H).
Embodiment 3
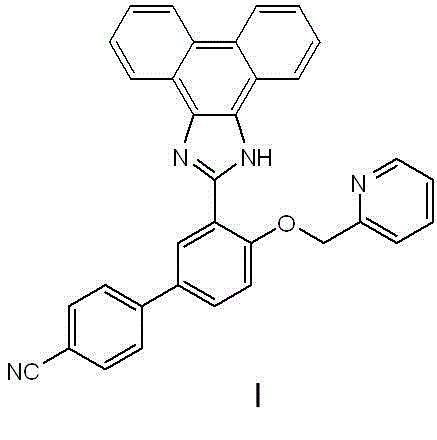
[0037] Embodiment 3, Synthesis experiment of fluorescent probe I of the present invention
[0038] Take 0.04 g of phenanthrenequinone and 0.16 g of ammonium acetate in 15 ml of ethanol, and then add 1.5 ml of dichloromethane. After complete dissolution, cool slightly, add sample III and a drop of acetic acid. After reacting for 2 hours, filter with suction and wash with ethanol to obtain the product probe Ⅰ. H NMR spectrum: 1 H NMR (400 MHz, CDCl 3) δ ppm : 12.97 (s, 1H), 9.00 (t, 2H, J=2.49), 8.34 (d, 1H, J=7.65), 8.79 (d, 1H, J=7.97), 8.74 (d, 1H, J=8.18), 8.39 (d, 1H, J=7.65), 7.55-7.90 (m, 10H), 7.42-7.48 (m, 2H), 7.24 (d, 1H, J=8.64), 5.53 (s, 2H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com