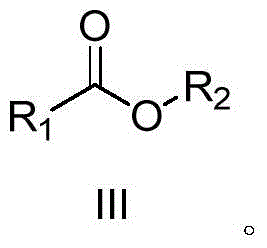
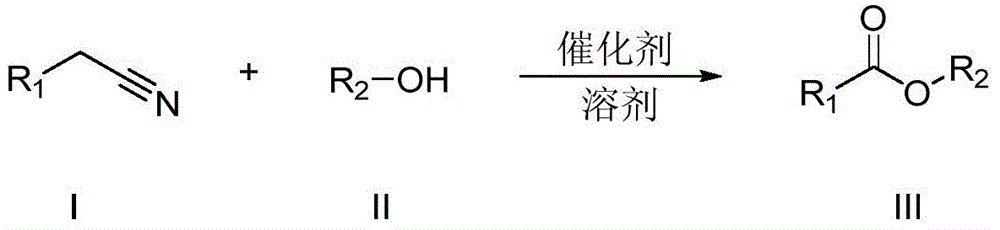
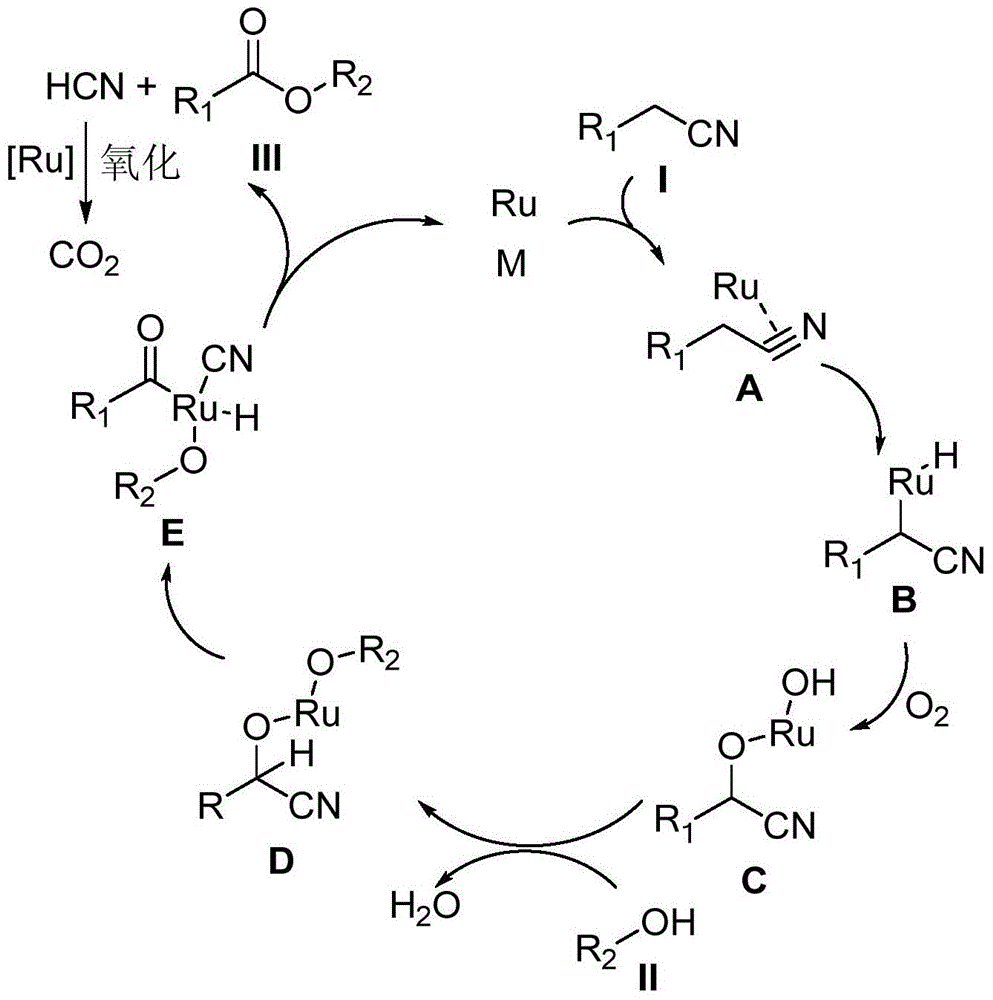
A kind of synthetic method of carboxylic acid or ester compound
A technology of ester compound and synthesis method, which is applied in the field of synthesis of carboxylic acid or ester compound, can solve the problems of difficult separation, non-recycling, large amount of condensing agent, etc., achieve mild reaction conditions, save production cost, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of methyl benzoate (III-1)
[0027] The reaction formula is as follows:
[0028]
[0029] Under air atmosphere, add 0.005g (2.5‰mmol Ru) Ru / C catalyst to the reaction flask, then add 10mL methanol (II-1), and then add 117μL (1mmol) benzylacetonitrile (I-1) under stirring. Add to the reaction flask, keep the temperature at about 30°C, stir and react for 37 hours, monitor the reaction, and the raw material (I-1) has basically reacted completely. After the reaction is completed, filter, wash the filter residue twice with methanol, combine the filtrate, dry, concentrate, and thin-layer chromatography (ethyl acetate / petroleum ether = 1 / 10) to obtain methyl benzoate represented by formula (III-1) The ester is 0.125g, the yield is 92.2%, and the purity is 98%. The structure of compound formula (III-1) is characterized as follows:
[0030] 1 H NMR(600MHz, CDCl 3 )δ8.05-8.03(m,2H),7.56-7.54(m,1H),7.44-7.43(m,2H),3.88(s,3H); GC-MS(EI): m / z 136[M + ].
Embodiment 2
[0031] Example 2: Preparation of methyl benzoate (III-1)
[0032] Under air atmosphere, add 0.01g (5‰mmol Ru) Ru / C catalyst to the reaction flask, then add 10mL methanol (II-1), and then add 117μL (1mmol) benzylacetonitrile (I-1) under stirring. Add to the reaction flask, keep the temperature at about 30°C, stir and react for 26 hours, monitor the reaction, and the raw material (I-1) basically reacts completely. After the reaction is completed, filter, wash the filter residue twice with methanol, combine the filtrate, dry, concentrate, and thin-layer chromatography (ethyl acetate / petroleum ether = 1 / 10) to obtain methyl benzoate represented by formula (III-1) The ester is 0.126 g, the yield is 93%, and the purity is 98%.
Embodiment 3
[0033] Example 3: Preparation of methyl benzoate (III-1)
[0034] Under air atmosphere, add 0.02g (10‰mmol Ru) Ru / C catalyst to the reaction flask, then add 10mL methanol (II-1), and then add 117μL (1mmol) benzylacetonitrile (I-1) under stirring. Add to the reaction flask, keep the temperature at about 30°C, stir and react for 20 hours, monitor the reaction, and the raw material (I-1) basically reacts completely. After the reaction is completed, filter, wash the filter residue twice with methanol, combine the filtrate, dry, concentrate, and thin-layer chromatography (ethyl acetate / petroleum ether = 1 / 10) to obtain methyl benzoate represented by formula (III-1) The ester is 0.130 g, the yield is 94.5%, and the purity is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com